Scientists from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr have developed an immobilization strategy using a polymer matrix that stabilizes a bio-inspired molecular catalyst.
The matrix was synthesized at the Ruhr University in Bochum (Germany), and the catalyst designed at Pacific Northwest National Laboratory in Richland (USA).
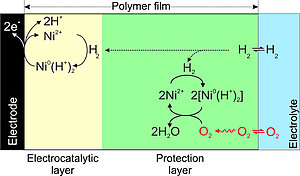
This catalyst is one of the most efficient hydrogen oxidation molecular catalysts, operating with low energy losses and high activity and could be an alternative to expensive metals like platinum. Its design has been inspired by nature, mimicking some features used by hydrogenases, the enzyme that catalyzes hydrogen production or oxidation using earth-abundant-metals like nickel and iron. A problem is that the catalyst is very oxygen sensitive on an electrode surface under fuel cell operating conditions, and the activity is completely lost after several hours of operation.
The here described polymer matrix creates a self-defense mechanism that extends its stability even in the presence of oxygen. This collaborative work has recently been published in <link https: www.nature.com articles s41467-018-03011-7 _blank external-link-new-window internal link in current>Nature Communications and has just been <link https: www.nature.com collections dmmhtcypsc _blank external-link-new-window internal link in current>highlighted by the editors.