Vita
| Study of Chemistry | Freie Universität Berlin (1969 - 1974) | ||
| Dr. rer. nat | Freie Universität Berlin (1977) | ||
| Habilitation | Freie Universität Berlin (1982) | ||
| Research Fellow | University of California, San Diego (1983 - 1984) | ||
| Assoc. Professor | Organic Chemistry FU Berlin (1986 - 1989) | ||
| Assoc. Professor | Experimental Physics, University Stuttgart (1989 - 1991) | ||
| Full Professor | Physical Chemistry, Max-Volmer-Institut, TU Berlin (1991 - 1999) | ||
| Honorary Professor | Heinrich-Heine-Universität Düsseldorf (since 2000) | ||
| Member | Max Planck Society (since 2000) | ||
| Director | MPI for Bioinorganic Chemistry; today: MPI CEC (2000-2017) |
Publications
Full Publicationslist
Selected Publications
- F. Lendzian, M. Huber, R.A. Isaacson, B. Endeward, M. Plato, B. Bönigk, K. Möbius, W. Lubitz and G. Feher The Electronic Structure of the Primary Donor Cation Radical in Rhodobacter sphaeroides R-26: ENDOR and TRIPLE Resonance Studies in Single Crystals of Reaction Centers Biochim. Biophys. Acta 1183, 139-160 (1993)
- W. Lubitz and G. Feher The Primary and Secondary Acceptors in Bacterial Photosynthesis III. Characterization of the Quinone Radicals Q-·A and Q-·B and by EPR and ENDOR Appl. Magn. Res. 17, 1-48 (1999)
- W. Lubitz, F. Lendzian, R. Bittl Radicals, Radical Pairs and Triplet States in Photosynthesis Acc. Chem. Res. 35, 313-320 (2002)
- M. Brecht, M. van Gastel, T. Buhrke, B. Friedrich, W. Lubitz Direct Detection of a Hydrogen Ligand in the [NiFe] Center of the Regulatory H2-Sensing Hydrogenase from Ralstonia eutropha in its Reduced State by HYSCORE and ENDOR Spectroscopy J. Am. Chem. Soc. 125, 13075-13083 (2003)
- A. Savitsky, A. A. Dubinskii, M. Flores, W. Lubitz, K. Möbius Orientation-resolving Pulsed Electron Dipolar High-field EPR Spectroscopy on Disordered Solids: I. Structure of Spin-correlated Radical Pairs in Bacterial Photosynthetic Reaction Centers J. Phys. Chem. B 111, 6245-6262 (2007)
- L. V. Kulik, B. Epel, W. Lubitz, J. Messinger Electronic Structure of the Mn4OxCa Cluster in the S0 and S2 States of the Oxygen-evolving Complex of Photosystem II Based on Pulse 55Mn-ENDOR and EPR Spectroscopy J. Am. Chem. Soc. 129, 13421 -13435 (2007)
- A. Silakov, E. J. Reijerse, S. P. J. Albracht, E. C. Hatchikian, W. Lubitz The Electronic Structure of the H-cluster in the [FeFe]-hydrogenase from Desulfovibrio desulfuricans: A Q-band 57Fe-ENDOR and HYSCORE Study J. Am. Chem. Soc. 129, 11447-11458 (2007)
- A. Silakov, B. Wenk, E.J. Reijerse, W. Lubitz 14N HYSCORE Investigation of the H-cluster of [FeFe] Hydrogenase: Evidence for a Nitrogen in the Dithiol Bridge Phys. Chem. Chem. Phys. 11, 6592 – 6599 (2009)
- N. Cox, H. Ogata, P. Stolle, E.J. Reijerse, G. Auling, W. Lubitz A Tyrosyl-Dimanganese Coupled Spin System is the Native Metalloradical Cofactor of the R2F Subunit of the Ribonucleotide Reductase of Corynebacterium ammoniagenes J. Am Chem. Soc. 132, 11197–11213 (2010)
- M.E. Pandelia, W. Nitschke, P. Infossi, M.-T. Giudici-Orticoni, E. Bill, W. Lubitz Characterization of a Unique [FeS] Cluster in the Electron Transfer Chain of the Oxygen Tolerant [NiFe] Hydrogenase from Aquifex aeolicus P. Natl. Acad. Sci. USA, 108, 6097-6102 (2011)
- D.A. Pantazis, W. Ames, N. Cox, W. Lubitz, F. Neese Two Interconvertible Structures Explain the Spectroscopy of the Oxygen Evolving Complex in the S2 State Angew. Chem. Int. Ed. 51, 9935 –9940 (2012) Angew.Chem. 124, 10074 –10079 (2012)
- L. Rapatskiy, N. Cox, A. Savitsky, W. Ames, J. Sander, M. Nowacyzk, M. Rögner, A. Boussac, F. Neese, J. Messinger, W. Lubitz Detection of the Water Binding Sites of the Oxygen-evolving Complex of Photosystem II Using W-band 17O Electron-Electron Double Resonance Detected NMR Spectroscopy J. Am. Chem. Soc. 134, 16619-16634 (2012)
- G. Berggren, A. Adamska, C. Lambertz, T. Simmons, J. Esselborn, M. Atta, S. Gambarelli, J.M. Mouesca, E.J. Reijerse, W. Lubitz, T. Happe, V. Artero, M. Fontecave Biomimetic Assembly and Activation of [FeFe]-Hydrogenases Nature 499, 66–69 (2013)
- J. Esselborn, C. Lambertz, A. Adamska-Venkatesh, T. Simmons, G. Berggren, J. Noth, J. Siebel, A. Hemschemeier, V. Artero, E.J. Reijerse, M. Fontecave, W. Lubitz, T. Happe Spontaneous Activation of [FeFe]-hydrogenases by an inorganic [2Fe] Active Site Mimic Nat. Chem. Biol. 9, 607-609 (2013)
- W. Lubitz, H. Ogata, O. Rüdiger, E.J. Reijerse Hydrogenases Chem. Rev. 114, 4081-4148 (2014)
- N. Plumeré, O. Rüdiger, A. A. Oughli, R. Williams, J. Vivekananthan, S. Pöller, W. Schuhmann, W. Lubitz A Redox Hydrogel Protects Hydrogenase from High Potential Deactivation and Oxygen Damage Nat. Chem. 6, 822–827 (2014)
- N. Cox, M. Retegan, F. Neese, D.A. Pantazis, A. Boussac, W. Lubitz Electronic Structure of the Oxygen-evolving Complex in Photosystem II Prior to O-O Bond Formation Science 345, 804-808 (2014)
- H. Ogata, K. Nishikawa, W. Lubitz Hydrogens Detected by Subatomic Resolution Protein Crystallography in a [NiFe] Hydrogenase Nature 520, 571-574 (2015)
- V. Krewald, M. Retegan, N. Cox, J. Messinger, W. Lubitz, S. DeBeer, F. Neese, D.A. Pantazis Metal Oxidation States in Biological Water Splitting Chem. Sci. 6, 1676-1695 (2015)
- F. Wang, R. Büchel, A. Savitsky, M. Zalibera, D. Widmann, S. E. Pratsinis, W. Lubitz, F. Schüth In situ EPR Study of the Redox Properties of CuO-CeO2 Catalysts for the Preferential CO Oxidation (PROX) ACS Catal. 6, 3520-3530 (2016)
- C. Sommer, A. Adamska-Venkatesh, K. Pawlak, J. Birrell, E. Reijerse, W. Lubitz Proton Coupled Electron Transfer whithin the H-Cluster as an Essential Step in the Catalytic Cycle of [FeFe] Hydrogenases J. Am. Chem. Soc. 139, 1440-1443 (2017)
- E. Reijerse, C. Pham, V. Pelmenschikov, R. Gilbert-Wilson, A. Adamska-Venkatesh, J. Siebel, L. Gee, Y. Yoda, K. Tamasaku, W. Lubitz, T. Rauchfuss, S. Cramer Direct Observation of an Iron-bound Terminal Hydride in [FeFe]-hydrogenase by Nuclear Resonance Vibrational Spectroscopy J. Am. Chem. Soc. 139, 4306-4309 (2017)
- S. Rumpel, E. Ravera, C. Sommer, E. Reijerse, C. Farés, C. Luchinat, W. Lubitz 1H NMR Spectroscopy of [FeFe] Hydrogenase: Insight into the Electronic Structure of the Active Site J. Am. Chem. Soc. 140, 131-134 (2018)
- S. Rumpel, C. Sommer, E. Reijerse, C. Farès, W. Lubitz Direct Detection of the Terminal Hydride Intermediate in [FeFe] Hydrogenase by NMR Spectroscopy J. Am. Chem. Soc. 140, 3863-3866 (2018)
- K. Möbius, W. Lubitz, N. Cox, A. Savitsky Biomolecular EPR Meets NMR at High Magnetic Fields Magnetochemistry, 4, 50, doi: 10.3390/magnetochemistry4040050 (2018)
Full Publicationslist
Functions
- Board Council Gesellschaft Deutscher Naturforscher und Ärzte e.V. (GDNÄ), expert representative Chemistry Group (since 2017)
- Member of the Editorial Board of Energy & Environmental Sciences (since 7/2016)
- Vice President of the Council of the Lindau Nobel Laureate Meetings (since 2015)
- Chair, Gordon Research Conferences on "Renewable Energy: Solar Fuels", 13.-18.05.2012, Lucca (Barga), Italy
- Vice Chair, Gordon Research Conferences on "Renewable Energy: Solar Fuels", 16.-21.01.2011, Ventura, CA (USA)
- Managing Director of the MPI for Bioinorganic Chemistry (2004-2011)
- Member of the International Advisory Board of the University of East Anglia Energy Materials Laboratory (since 2008)
- Chairman of the Advisory Board of the Farkas Minerva Advisory Board; Jerusalem (2007-2013)
- President of the International EPR/ESR Society (2005-2008)
- Member of the Council of the Nobel Laureate Meetings in Lindau (since 2004)
- Member of the Advisory Board Physical Chemistry Chemical Physics (since 2011)
- Member of the Advisory Board Biopolymers: Biospectroscopy (since 1995)
- Member of the Editorial Advisory Board Journal of Biological Inorganic Chemistry (since 1999)
- Member of the Advisory Board of Applied Magnetic Resonance (since 2001)
- Member of the Advisory Board Energy & Environmental Science (since 2007)
- Member of the International Advisory Board for the International Conference on Clean Energy (2011)
- Member of the Editorial Advisory Board of the Book Series "Sustainable Energy Developments" (CRC Press)
Awards
- Fellow of the International EPR Society 2017
- Robert Bunsen Lecture, German Bunsen Society for Physical Chemistry (2017)
- Doctorat honoris causa, Université d'Aix-Marseille, France (2014) - Prof. Dr. Dr. h. c. Dr. h.c. Wolfgang Lubitz
- Foreign Member of the Academy of Sciences of the Republic of Tatarstan (2012)
- Fellow of ISMAR (International Society of Magnetic Resonance) Fellow (2010)
- Honorary doctorate Dr. h. c., Uppsala University, Sweden (2008) - Prof. Dr. Dr. h. c. Wolfgang Lubitz
- Gold Medal of the International EPR Society (2005)
- Fellow of the Royal Society of Chemistry. U.K. (2004)
- Bruker Prize, Royal Society of Chemistry, ESR group, U.K. (2003)
- International Zavoisky Award, Russian and Tatarstan Academy of Sciences, Kazan, Russia (2002)
- Max-Kade-Fellowship, New York (1983)
- Otto-Klung-Preis für Chemie, FU Berlin (1978)
Research - Biophysical Chemistry
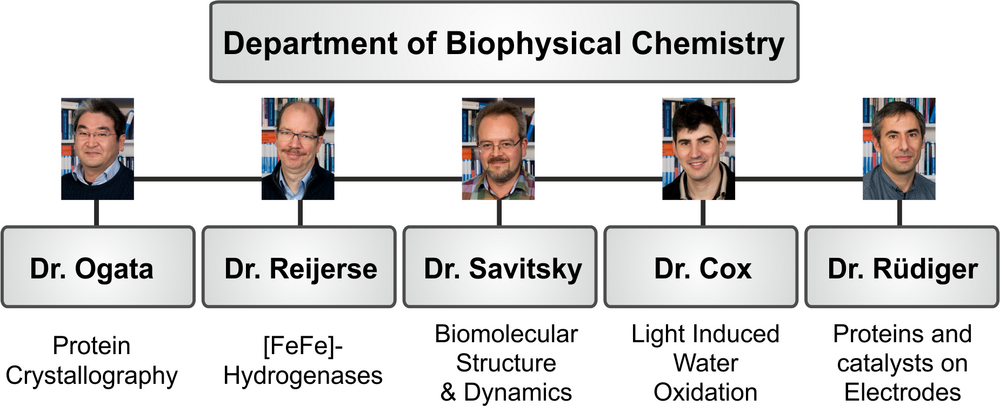
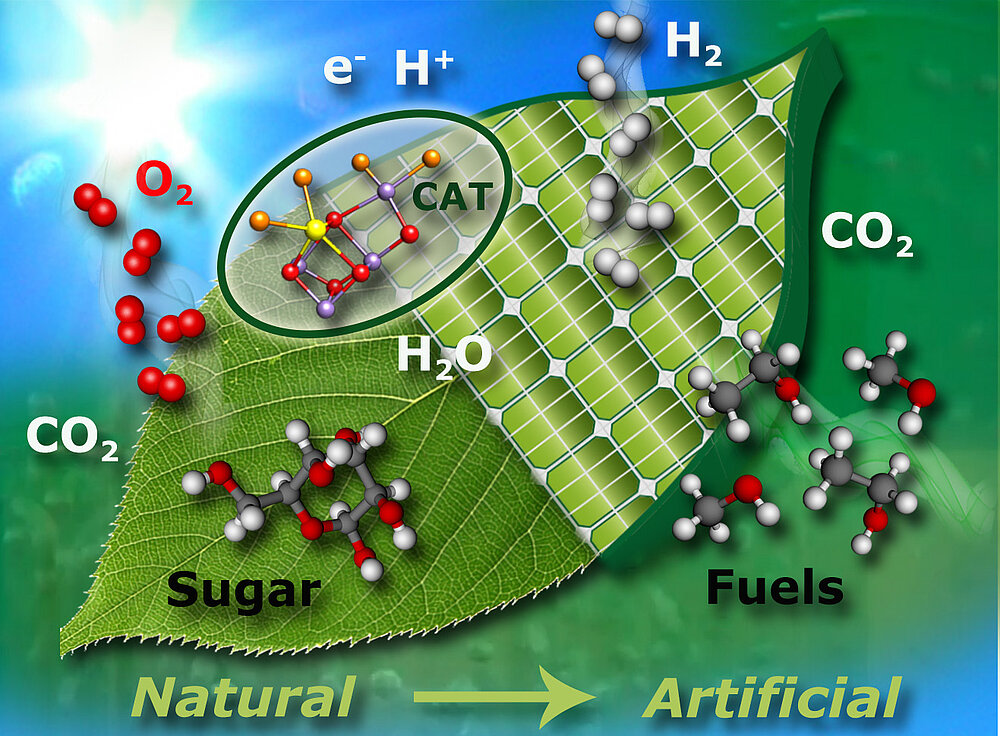
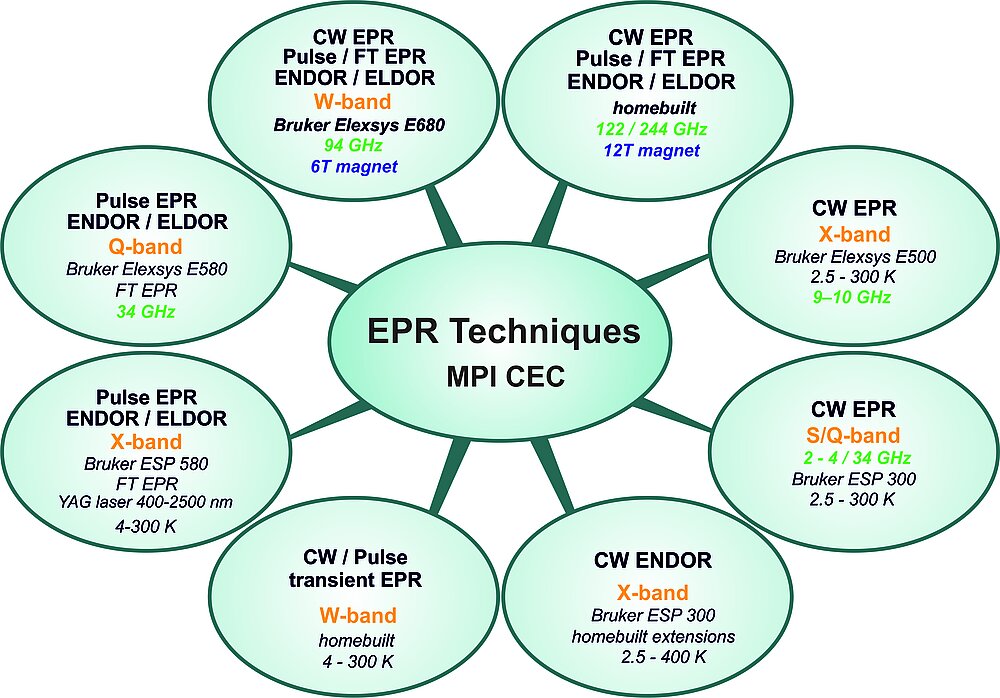
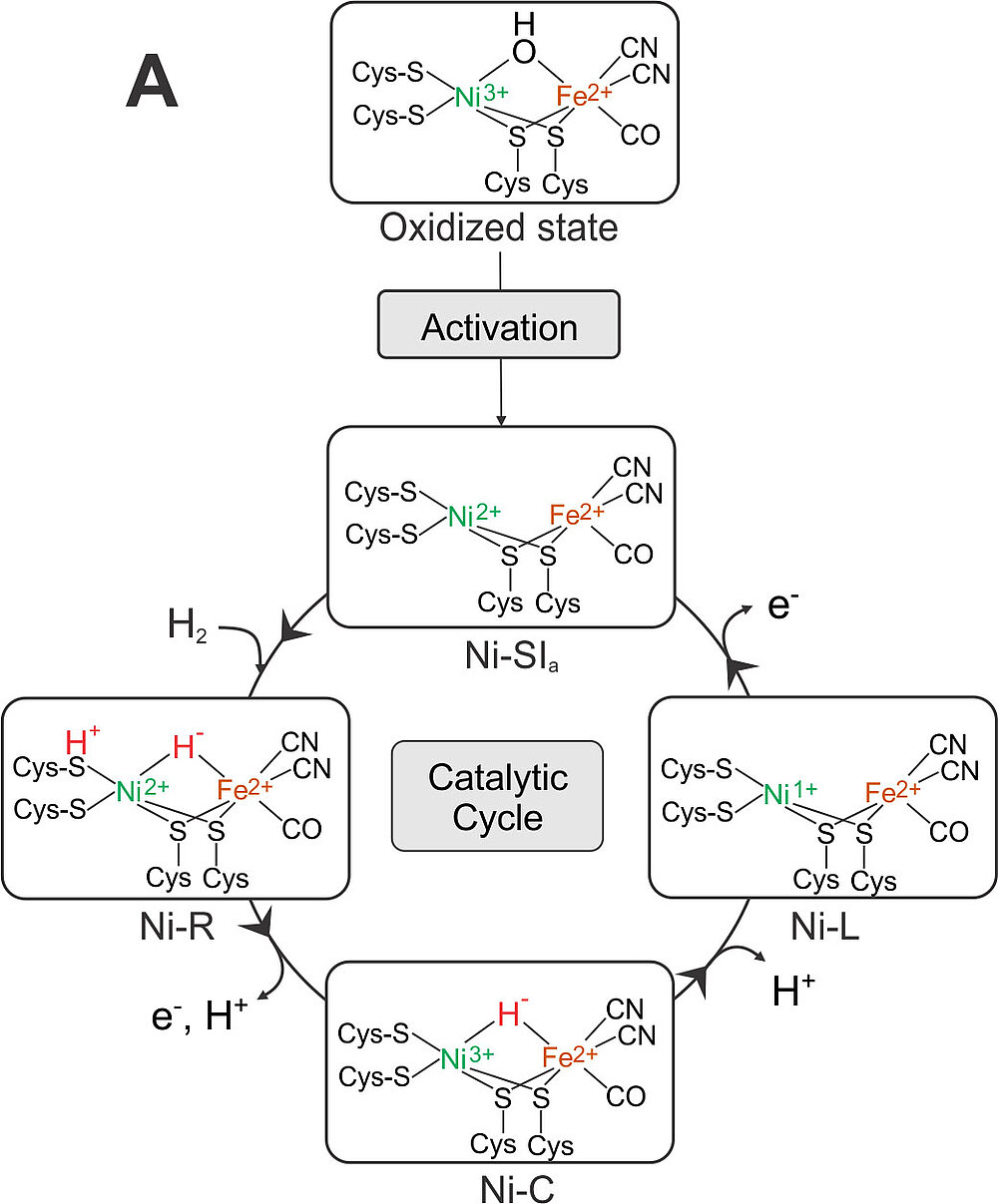
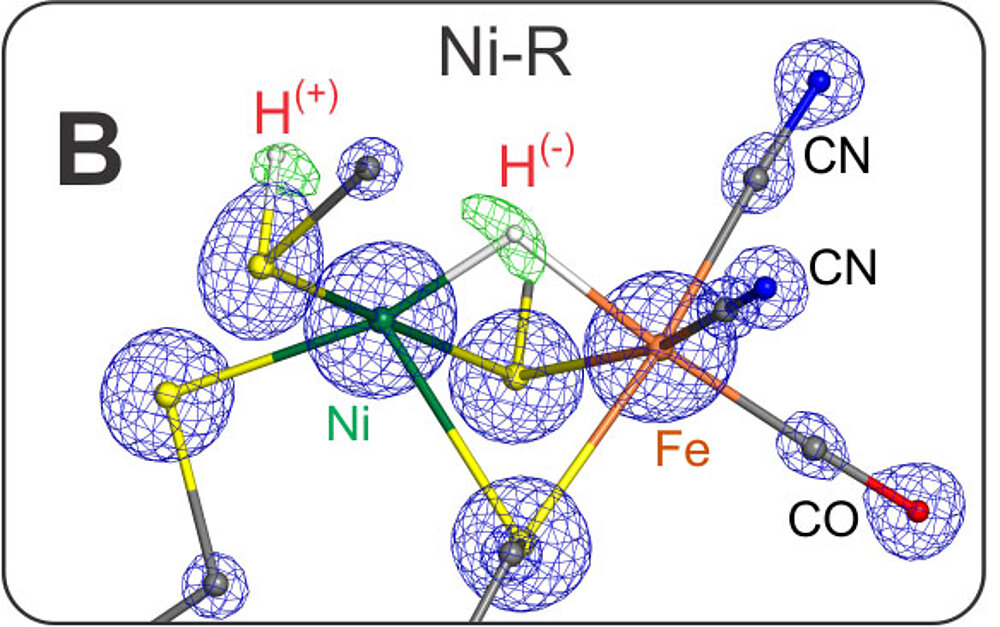
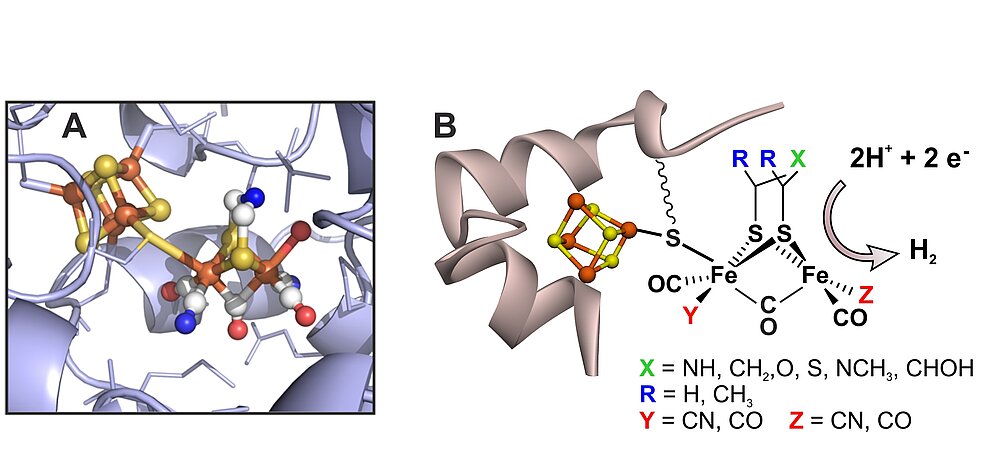
Fig. 4: (A) The H-cluster of the active site of [FeFe] hydrogenases in its protein surrounding, showing the two subclusters [4Fe-4S]H (left) and [2Fe]H (right); (B): picture showing a selection of non-native active site mimics of the [2Fe]H -subcluster that could be introduced into the apo-protein (carrying only the [4Fe-4S]H -subcluster) via artificial maturation of the hydrogenase from Chlamydomonas reinhardtii; for details see [30].
The Department of Biophysical Chemistry
The department of Biophysical Chemistry has been established in 2000 by Prof. Wolfgang Lubitz after his appointment as a director of the Max Planck Institute for Bioinorganic Chemistry, formerly Radiation Chemistry, which in 2012 was renamed Max Planck Institute for Chemical Energy Conversion (MPI-CEC). At present there are approximately 30 scientists, postdocs, doctoral students and technicians working in the department. Five research groups, each headed by a group leader, are working on specific topics (scheme 1).
“Artificial photosynthesis”, the concept that is explored in the department, is illustrated in Figure 1. More specifically, we are studying native systems (enzymes) to understand the ingenious concepts of Nature with the aim to use this knowledge to design and synthesize chemical systems for efficient light energy capturing, energy conversion and storage in chemical compounds. In this broad research field we are focusing on two biological systems that contain complex transition metal catalysts in their active sites: i) the water oxidizing complex (WOC) - or oxygen evolving complex (OEC) - of oxygenic photosynthesis; and ii) hydrogenases that produce or convert molecular hydrogen. We use a variety of different physical techniques to study these systems, including X-ray crystallography, X-ray spectroscopy, Mössbauer spectroscopy, magnetic resonance (EPR/NMR), optical and vibrational spectroscopy as well as electrochemistry. Particular emphasis is placed on characterizing the magnetic properties of the metal sites and their ligand sphere, for which advanced electron paramagnetic resonance (EPR) techniques are employed. Most of the investigated systems are prepared in-house, allowing sample optimization and modification in an efficient way. The information obtained from such spectroscopies is supplemented by modern quantum chemical approaches, with the aim of understanding the catalytic function at the atomic level (cooperation with Dr. Pantazis, Molecular Theory and Spectroscopy Department of Prof. Neese). In this way, insight into enzymatic water splitting and hydrogen production and consumption in Nature has been obtained, providing new and important design criteria for synthetic bioinspired catalysts for energy conversion (cooperation with the Department Schlögl, Heterogeneous Catalysis). Our recent work has been summarized in overview articles on water oxidation [1-3] and hydrogenases [4].
Instrumentation and Methodologies
A central mission of our department is the development of new instrumentation, methodologies and experimental techniques. In this endeavor we are closely working together with the technicians, engineers and the excellent workshops of the institute. We are recognized as a center for multifrequency EPR and related spectroscopies. The 8 major instrumental EPR stations (Scheme 2) span the microwave frequency range from 2 to 244 GHz at fields between 0 and 12 T, operating in both CW and pulse mode configurations and at temperatures from 1.5 K to significantly above room temperature. To become independent of liquid helium supply we have started to replace all cryostats (for sample cooling) and high field magnets with cryogen-free systems. Accessories for additional MW channels and RF (NMR) irradiation for multiple resonance experiments are available as well as an arbitrary wave form generator (AWG) for pulse shaping and multifrequency MW pulse sequences. Furthermore we are building our own probe heads (MW resonators) adapted to specific applications. Most instruments allow in-situ laser irradiation for studying light-dependent processes. Short-lived paramagnetic species can be studied down to lifetimes of a few nanoseconds. Recently high pressure equipment has been set up that allows high field EPR experiments to be performed at up to 2.5 kbar (4 kbar peak).
These developments in the MPI together with improvements in related microwave technology and data acquisition have led to a substantial increase in the sensitivity and stability of the EPR measurements. This has dramatically expanded the array of chemical systems that can be characterized using EPR techniques, including large protein supercomplexes with multiple paramagnetic centers, fast relaxing spin systems such as transition metal complexes and related materials, species with higher spin states and samples with constrained dimensions, e.g. small single crystals - to name only a few. EPR experiments on single crystals are of particular importance; in the department we are therefore running a crystal growth laboratory. Using microresonators we are now able to investigate very small single crystals with volumes of only a few nanoliters. Short-lived intermediates in reaction cycles (lifetimes of a few milliseconds) can be trapped by a recently implemented rapid freeze-quench system developed in our laboratory. This has been successfully employed for trapping several intermediates (S-states) in the light-driven water splitting clockwork of PS II using laser excitation combined with fast injection of 17O labeled water in the catalytic cycle (Kutin/Cox et al., to be published).
Another important recent development is the construction of a chemical reactor engineered inside an EPR resonator, allowing the in situ real time detection of the active species in a reaction using a heterogeneous catalyst. This station is combined with analytic gas analysis of the reaction products [5].
Particular emphasis is placed in our EPR laboratory on methods that are able to resolve the electron-nuclear hyperfine couplings between the electron spin and the nuclear spin(s) that are delivering a map of the spin density distribution of the system – unique information that is only available by advanced EPR methods. Next to the more established techniques, electron spin echo modulation (ESEEM) and electron-nuclear double resonance (ENDOR), we have developed and used electron-electron double resonance- (ELDOR) detected NMR (EDNMR) at a range of mw frequencies together with simulation software. In this way even for magnetic nuclei with high nuclear spin like 61Ni, 55Mn, 17O and 14N the hfcs could be measured in complex metallobiomolecules with excellent sensitivity. The methodology has been described in recent articles by our group [6-8].
During the last two years we have started to use NMR techniques to determine the structure of metalloproteins in solution. First results have been obtained and published on the ferredoxin (petF) from a green algae that acts as shuttle between the photosynthetic electron transport and other proteins, e.g. hydrogenase (producing hydrogen) [9]. In a subsequent publication we were able to determine a much more complete NMR structure by substituting the paramagnetic iron ions in the [2Fe-2S] cluster in petF by two gallium ions, that are diamagnetic [10]. Very recently we have also started to use paramagnetic NMR to study the active site of various genetically modified and/or labeled, artificially matured [FeFe] hydrogenases (Rumpel et al., to be published).
In recent years we have expanded our activities to obtain also vibrational data that often allow detection of all intermediates in a reaction cycle. For hydrogenase and also photosynthesis research FTIR techniques are very important. We have set up two instruments for experiments covering a wide temperature range (4K to 350K) with light access of the cryostat to follow photoinduced reactions. An ATR (attenuated total reflection) cell for surface IR experiments is also available. Spectroelectrochemistry can be performed in an optical transparent thin layer electrochemical (OTTLE) cell. A similar cell is also used for UV-VIS detection of redox processes for which we have a separate instrument. Furthermore time resolved FTIR experiments are possible in step-scan mode performed on our second FTIR instrument. Another smaller FTIR instrument is placed in a glove box for surface enhanced infrared absorption (SEIRA) spectroscopy in combination with electrochemical control. In the department of Frank Neese a sensitive new Raman instrument has been built that will be used for joint Resonance Raman (RR) experiments. Vibrational data have also been obtained using inelastic Mössbauer scattering (nuclear resonance vibrational spectroscopy, NRVS) in cooperation with Stephen Cramer (UC Davis) on a synchrotron using respective monochromators (<1 meV resolution) at the beamline [11, 12]. This technique allows detection of vibrations that are often not accessible by FTIR or RR, e.g. of metal-hydrides in larger proteins. In the optical regime a new sensitive MCD instrument is available in the Neese Department that we are using together with Eckhard Bill with whom we are also collaborating on Mössbauer spectroscopy [13]. X ray absorption (XAS) and emission (XES) experiments have been started together with Serena DeBeer in the MPI [14].
As mentioned above the extension of our electrochemistry facility has made great progress during the last 3 years. Next to the classical techniques (cyclic voltammetry, coulometry, spectroelectrochemistry etc.) protein film electrochemistry (PFE) can now be performed routinely under controlled environmental conditions in two glove boxes equipped with gas mixers. Furthermore, we have successfully started to perform advanced EPR experiments on electrodes to follow the paramagnetic species and their conversion in the investigated processes (“electrochemical EPR”). This is particularly important for the detection of species (catalysts) on surfaces and embedded in polymer matrices [15-17].
In close collaboration with the Department of Prof. Neese, DFT and ab initio calculations are being performed to relate measured spectroscopic parameters to structure and obtain reliable, unified electronic and geometrical structural models, for example of catalytic intermediates, important for the elucidation of reaction mechanisms, see [11, 18, 19].
Collaborations within the MPI CEC, the Mülheim Chemistry Campus and with Neighboring Universities
Between our Biophysical Chemistry Department and the Molecular Theory and Spectroscopy Department of Frank Neese exist very close and successful ongoing collaborations both for the water oxidation project and the hydrogenases, which is demonstrated by a large number of joint publications. Water oxidation is studied with spectroscopic techniques (Dr. N. Cox) and theoretical methods (Dr. D. Pantazis); the joint effort has led to an atomic level understanding of the structure and the structural changes of the water splitting unit, including an assignment of oxidation and spin states of the individual manganese ions in each of its metastable transition states, as well as an assignment of the sites of substrate water binding, the process of O-O bond formation and O2 release [1-3, 18-20]. Collaboration with Dr. M. van Gastel has led to a significant advancement in the understanding of the structure and function of the catalytic site in [NiFe] hydrogenases [21]. Together with Dr. E. Bill, new important information using Mössbauer and EPR spectroscopy has been obtained for the regulatory hydrogenase, a sensor protein [13]. The work has been complimented by XAS and XES measurements on [NiFe] model systems with Prof. S. DeBeer [14]. With Dr. T. Weyhermüller we are collaborating to synthesize model systems both for water oxidation (manganese clusters) and particularly for hydrogen production [22]. Together with the recent ultra-high resolution crystallographic studies in our department [23] a near complete understanding of the [NiFe] hydrogenase catalytic cycle is within reach.
With the Department Schlögl Heterogeneous Catalysis we have started to set up EPR experiments to detect paramagnetic species in photocatalysis. A close collaboration in a joint project on manganese clusters on surfaces and in solution has been established within the BMBF project “Verbundvorhaben MANGAN” (principal investigators: S. DeBeer, N. Cox, D. Pantazis, A. Knop-Gericke). With Dr. S. Becker’s group functionalized carbon nanotubes for MnO electrocatalysis have been investigated with EPR. With the independent research group leader Dr. Jennifer Strunk (MPI CEC/Universität Duisburg/Essen) an EPR characterization of surface doped TiO2 particles for photocatalysis has been performed.
With the neighboring MPI für Kohlenforschung we have an ongoing collaboration with Dr. C. Farès to obtain protein structures in solution using NMR techniques [9, 10]. We have also initiated a project with Prof. F. Schüth/Dr. Ryan Wang on in situ EPR detection of the catalytically active sites in CuO/CeO2 for the preferential gas phase oxidation of CO to CO2 [5]. This novel technique is highly interesting also for other similar catalytic reactions, e.g. the water-gas shift reaction that is currently under study. Within the Cluster of Excellence (RU Bochum) we are also collaborating with the theory group of Prof. W. Thiel on protein dynamics including the solvent shell (QM/MM) [24].
Since 2012 Prof. W. Lubitz is PI in the Cluster of Excellence “RESOLV”
Hydrogenases
Hydrogenases are Nature´s catalysts for the oxidation of molecular hydrogen or the reverse reaction, the production of H2 from protons. A basic understanding of the structure, function and dynamics of this class of enzymes is of key importance for a future biologically based hydrogen production technology and for the design and synthesis of bioinspired model systems for hydrogen conversion or production catalysis. In our department both the [NiFe] and the [FeFe] hydrogenases are studied along with appropriate model systems in a combined effort of different groups and by applying a broad range of physical techniques.
[NiFe] Hydrogenase: During the last 3 years the structures of all intermediates in the activation path and catalytic cycle of [NiFe] hydrogenases were finally resolved. The result is shown in Figure 3.
The active species is Ni-SIa, a diamagnetic complex, Ni(II)Fe(II), with an open bridge between nickel and iron - prepared structurally and electronically to take up H2. Ni-SIa has been characterized using FTIR and Mössbauer spectroscopy [13]. This species attaches dihydrogen to yield Ni-R. We have obtained an ultrahigh resolution X-ray crystallographic structure (0.89 Å resolution) [23] of Ni-R in single crystals of the [NiFe] hydrogenase from Desulfovibrio vulgaris Miyazaki F, in which the product of heterolytic splitting of H2 (H+ and H-) could directly be identified; the hydride is sitting in the bridge between Ni and Fe (closer to Ni) and the proton is attached to the sulfur of one of the terminal cysteines next to the putative proton transfer canal. Furthermore, over 90% of the other hydrogens at ligating cysteines and surrounding amino acids near the active center could be detected including some water molecules.
In this structure the CO and CN- ligands could be distinguished for the first time and the proton transfer channel mapped out. Furthermore, the hydrogen bond network is available in unprecedented precision since the crucial hydrogens could directly be seen in many cases. The loss of an electron and a proton leads to Ni-C that only carries the hydride bridge, as shown in our earlier EPR work [25] and corroborated by DFT calculations on a large model of the active site together with the Neese group [26]. Ni-C is converted back to Ni-SIa by loss of a proton and an electron, most probably in two steps (via a Ni-L like state). The sequence of these PCET reactions is not yet fully elucidated; it may involve several intermediates, (Ni-L and Ni-R states). The reductive activation of the oxidized state (Ni-B/Ni-A) to Ni-SIa is done by loss of water (protonation of the OH- bridge, see Figure 3). The spectroscopic signatures of the two oxidized states present in standard [NiFe] hydrogenases could also be explained by EPR and FTIR experiments together with QC models [21].
[FeFe] Hydrogenase: As discussed above the catalytic H2 splitting and H2 formation in [NiFe] hydrogenases is performed by the nickel ion aided by a terminal cysteine sulfur acting as a base. In the [FeFe] hydrogenases an iron ion is employed in concert with a special azadithiolate ligand bridging the two irons, in which the nitrogen is functioning as a base (see Figure 4). The existence of the nitrogen, first shown by pulse EPR and ENDOR in our group [27], was finally proven by the insertion of several biomimetic complexes into the apo-protein of bacterial and algal [FeFe] hydrogenases. This seminal work has been done together with M. Fontecave (Paris), V. Artero (Grenoble) and T. Happe (Bochum) and was published in 2013 [28, 29]. These first experiments on the artificial maturation of [FeFe] hydrogenases had far-reaching consequences for hydrogenase research, since the observed spontaneous cluster assembly could be used for obtaining larger amounts of hydrogenases, for isotopic labeling and changing metals and/or substituents or ligands, and to this end obtaining improved properties and function of the hydrogenase hybrids. Furthermore, the results could offer pathways to probe the theory of geobiochemical evolution of the hydrogenases and other related enzymes.
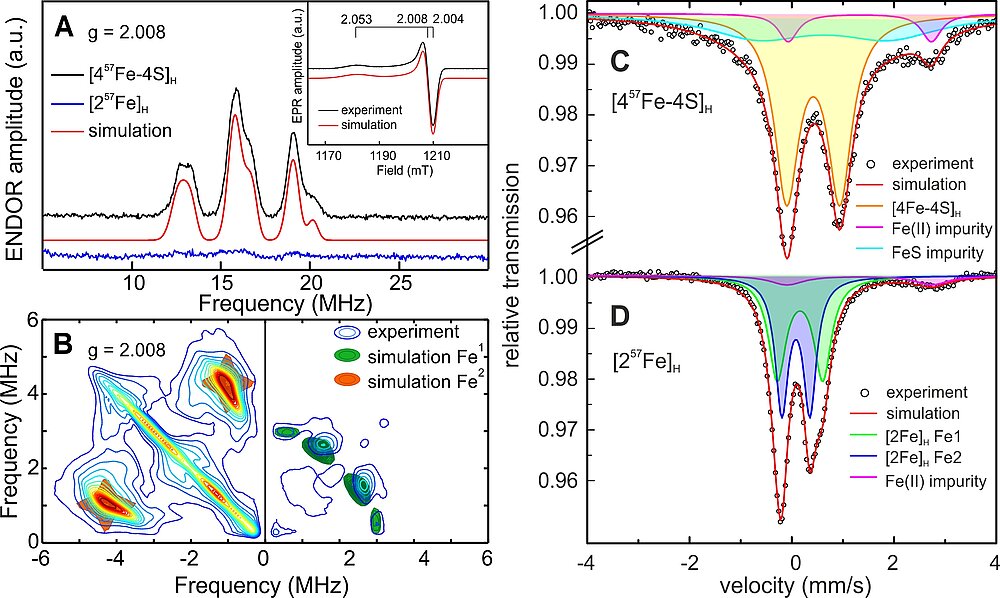
In Figure 4 a series of compounds with changed ligand sphere is shown that could be incorporated into the apo-protein. It is interesting that all complexes showed much lower activity than the native enzyme both for H2 oxidation and production; only the compound with one instead of two CN- ligands attained 50% activity of the natural system [30]. In Figure 5 an example is shown demonstrating how specific labeling can lead to new information. Here a hydrogenase with the native cofactor is investigated for which either the [4Fe-4S]H-cluster or the [2Fe]H-cluster is 57Fe labeled. This allows the separate detection and assignment of data from Mössbauer and 57Fe EPR/ENDOR spectroscopy for these subsites for different states in the catalytic states [31].
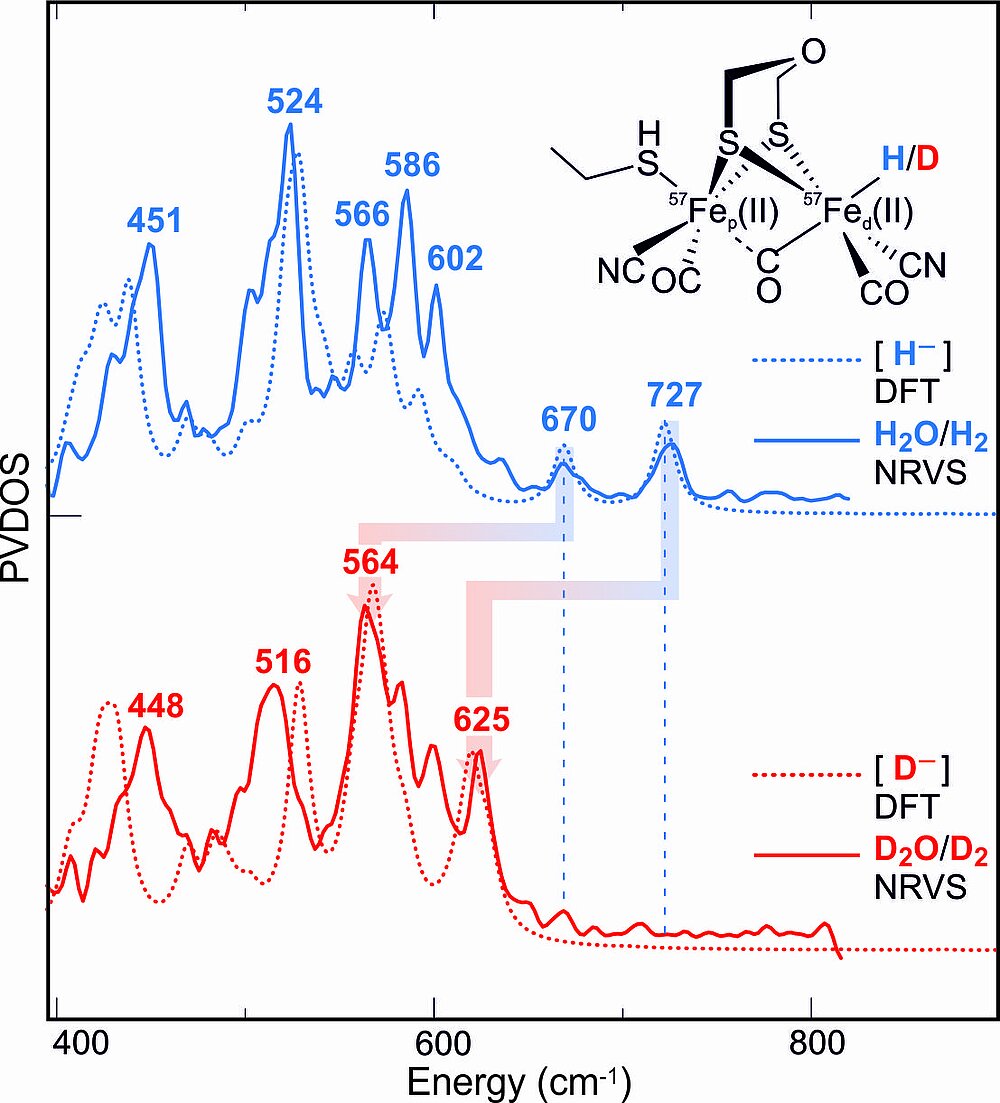
The specific 57Fe labeling of the [2Fe]H-subcluster also allowed the preparation (and stabilization) of the key intermediate that is carrying the hydride end-on at the distal Fe. For the detection vibrational spectroscopy (NRVS) at the synchrotron SPring8 (Hyogo, Japan) was used in collaboration with the group of S. Cramer (UC Davis), employing H/D exchange and a special cofactor that blocks proton transfer and stabilizes the transient intermediate, see Fig. 6 (Reijerse et al., submitted).
Furthermore, the availability of modified (hybrid) [FeFe] hydrogenases opened the possibility to detect and assign the proton nuclei of the active site using paramagnetic NMR, a collaborative research project with C. Farès (KOFO, Mülheim) and C. Luchinat (Florence) (Rumpel et al., in preparation).
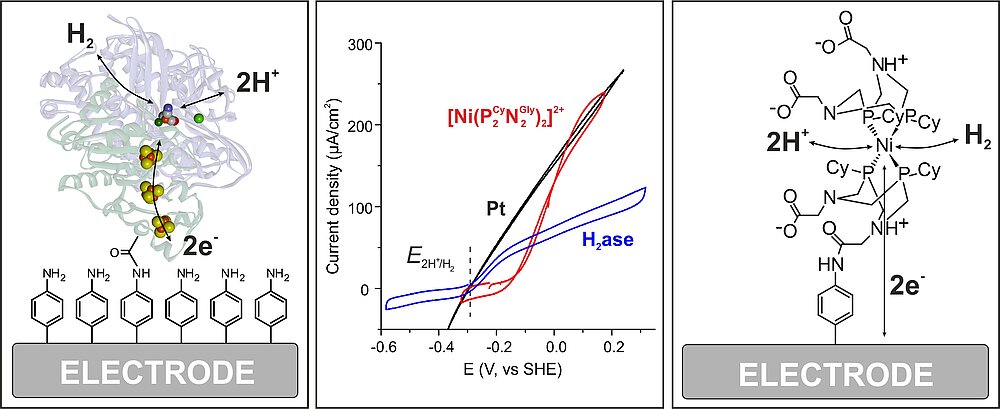
Electrochemistry of Hydrogenases: The oxygen sensitivity of the [NiFe] hydrogenases is a major drawback for practical applications in (bio)fuel cells or water electrolysis. Fortunately, several oxygen tolerant [NiFe] hydrogenases are known, e. g, from Knallgas bacteria. How these are dealing with the attack of O2 has been a topic of intense research in the past 5 years, see [4] for details. Together with the group of W. Schuhmann (RUB) we could show that a special designed smart matrix (a viologen-based polymer/hydrogel) is able to protect an embedded O2-sensitive [NiFe] hydrogenase against oxygen attack and high potential deactivation [15]. This approach therefore eliminates two of the major problems that prevented the use of hydrogenases in biotechnological devices. Recently we could show that such a protection can also be achieved with the much more O2-sensitive [FeFe] hydrogenases [16]. We currently work on active hydrogenase model systems in cooperation with Wendy Shaw (PNNL, USA). In Figure 7 cyclic voltammograms are shown for H2 reduction on Pt compared with [NiFe] hydrogenase and a model complex both covalently attached in the same way to the electrode. This demonstrates the excellent performance of both the native and model system - depending on experimental conditions [17].
Hydrogenase Model Systems: In recent years we have started to synthesize new structural and functional model systems for [NiFe] and [FeFe] hydrogenases and characterized them (for an overview see section 6 in ref. [4]). Interesting first results were the demonstration of a reversible protonation of sulfur in a NiFe-model, the design of a complex with Ni(low spin) Fe(high spin) and Fe as active site, and also a mono-iron compound with (PR2NR2)- and Cp*- ligands for H2 production, for which an Fe-hydride species could be clearly detected and characterized [22]. We have also performed a large number of spectroscopic experiments on hydrogenase model systems of other groups, see [32] for an example.
Wateroxidase
In photosystem II of oxygenic photosynthesis a protein-bound oxygen-bridged Mn4Ca cluster performs the process of light-induced water oxidation in a complex catalytic cycle, thereby releasing molecular oxygen, protons and electrons. The protons are used to form a gradient across the membrane driving ATP production, whereas the electrons are used to form NADPH; with both molecules (ATP, NADPH) subsequently used in the dark reactions (Calvin cycle) to reduce CO2 to carbohydrates. During water splitting the cluster passes through five intermediate states S0 to S4, where the subscript denoted the number of transiently stored oxidizing equivalents. For many decades, the lack of structural data, the absence of spectroscopic methods with high resolution and sensitivity, and the complexity of the water splitting reaction led to rather slow progress in this highly important research field. With the advent of atomic level X-ray structures in Japan (Shen et al.; Nature, 2011 and 2015), concomitant progress in X-ray spectroscopy and time-resolved X-ray diffraction in USA, and with the development of novel sensitive magnetic resonance techniques here in Mülheim, this field has witnessed an explosion of research activity in recent years. Together with theoretical calculations performed in the Department of Frank Neese an understanding of the process of light-induced water oxidation in Nature is now in sight.
In our department we have used mainly magnetic resonance techniques to study the trapped S-states of the water splitting cycle. Using advanced multifrequency pulse EPR, ENDOR and EDNMR techniques we were able to detect and characterize the flash-generated, freeze-trapped paramagnetic states S0, S2 and S3 (S1 is diamagnetic and S4 is a transient state). By a careful analysis – backed up by QC calculations – we determine the site oxidation and spin states of all Mn ions and their spin coupling for all intermediates of the catalytic cycle [18, 19].
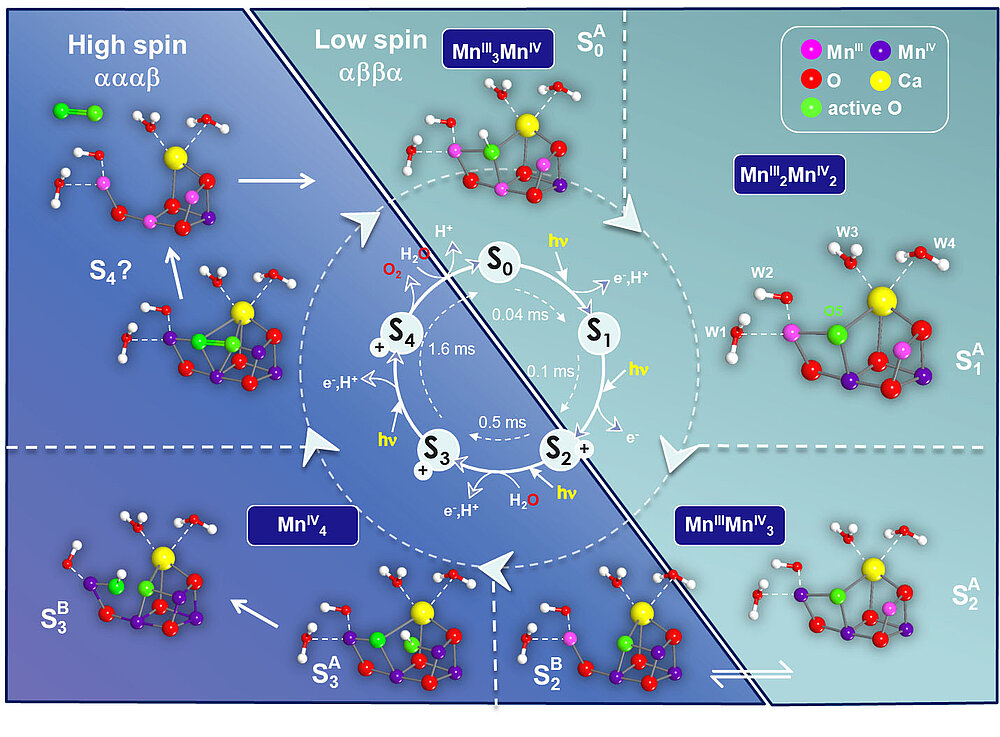
The binding of the first substrate water and its incorporation into the Mn-cluster had already been measured by us using magnetically labeled water (H217O) and detection by high field 17O EDNMR [33]. In more recent work the second water binding event was proposed to take place in the S2 → S3 state transition; S3 is the last metastable intermediate of the complex prior to O-O bond formation [20]. In the S3 state, the two substrate oxygens are ideally located, bound between two Mn sites, allowing highly efficient O-O bond formation in the final S4 state (Figure 8). This supports an oxo-oxyl coupling model originally proposed by Per Siegbahn (Stockholm).
Figure 8 summarizes the collected knowledge about the water oxidation cycle in oxygenic photosynthesis based on our joint spectroscopic and theoretical results in Mülheim. An interesting aspect is that in the S2 state two structural isomers have been found that allow a low-barrier transition from a low-spin SA2 (Stot = 1/2) to a high-spin SB2 (Stot = 5/2) configuration [34]. The two species are in full agreement with known EPR data. The high spin state, which is a consequence of a closed cubane configuration with the dangling Mn(III) having a vacant coordination site, enables formation of the S3 (Stot
References
[4] Lubitz, W., Ogata, H., Rüdiger, O. and Reijerse, E. (2014) Hydrogenases. Chem. Rev., 114, 4081.
[6] Cox, N., Lubitz, W. and Savitsky, A. (2013) W-Band ELDOR-Detected NMR (EDNMR) Spectroscopy as a Versatile Technique for the Characterization of Transition Metal-Ligand Interactions. Mol. Phys., 111, 2788.
[12] Wang, H., Yoda, Y., Ogata, H., Tanaka, Y. and Lubitz, W. (2015) A Strenuous Experimental Journey Searching for Spectroscopic Evidence of a Briding Nickel-iron-hydride in [NiFe] Hydrogenase. J. Synchrotron Rad., 22, 1334.
Vita
| Diplom in Chemie | Universität Heidelberg (1967) |
| Promotion | Universität Heidelberg, Institut für Anorganische Chemie, Prof. Hans Siebert (1969) |
| Assistent | Universität Heidelberg, Inst. für Anorganische Chemie, Labor Prof. Siebert und Prof. Weiss (1969-1972) |
| Post-doc | University of Leeds, GB, Prof. A. G. Sykes (1972-1973) |
| Habilitation | Universität Heidelberg (1974) |
| Privatdozent | Universität Heidelberg (1974-1975) |
| Professor, wiss. Rat | Technische Universität Hannover (1975-1981) |
| Professor | Lehrstuhl für Anorganische Chemie, Ruhr-Universität Bochum (1981-1994) |
| Founding Director | Max-Planck-Institut für Bioanorganische Chemie (1994-2010) |
| Emeritus | Max-Planck-Institut für Bioanorganische Chemie (2010) |
Publications
Awards
- Französisch-Deutscher Alexander von Humboldt, Gay Lussac Forschungspreis, April 1995
- Wilhelm-Klemm-Medaille, Gesellschaft Deutsche Chemiker, 2000
- John-Bailar-Medaille, University of Illinois, 2000
- Centenary Medal, Royal Society of Chemistry (London), 2002
- American Chemical Society Award in Inorganic Chemistry, 2006
- Ruhrpreis, 2005
Functions
- Gesellschaft Deutscher Chemiker
- Deutsche Akademie der Naturforscher, Leopoldina
- Auswärtiges Mitglied der Chemical Research Society of India
- Mitglied des Auswahlgremiums des Otto Hahn-Preises (Gesellschaft Deutscher Chemiker; Stadt Frankfurt)
- Vorsitzender des Gremiums zur Auswahl des Alfred-Stock-Gedächtnispreises (Gesellschaft Deutscher Chemiker)
- Fachgutachter der Deutschen Forschungsgemeinschaft, 8 Jahre
- Mitglied des Heisenbergausschusses, 6 Jahre
Research - Bioinorganic Chemistry
Bioinorganic Chemistry
The research of my group focuses on two areas of Inorganic Chemistry, namely Coordination Chemistry of Transition Metal Ions and Bioinorganic Chemistry.
Coordination Chemistry
We continue to extensively study the coordination chemistry of organic p radical ligands with paramagnetic transition metal ions. The main focus is the correct description of the electronic structure of compounds containing open-shell organic ligands and paramagnetic metal ions. In a very simplified picture we have to discern between the following two possibilities:
Mn+ — L or M(n-1)+ — L•
(1) (2)
The question arises if the two structures (1) and (2) represent
a) two mesomeric resonance structures of a single electronic ground state configuration or
b) two distinctly different ground states with differing chemical reactivities(equilibrium).
We have developed spectroscopic and theoretical methodology (in collaboration with F. Neese) to answer such questions. In fact, computational chemistry has been found to be an extremely powerful tool for the discovery of coordinated radicals. Broken symmetry DFT calculations of the electronic structure of a given compound and calculations of their spectroscopic properties(UV-vis IR-, RR-, EPR-, and Mössbauer spectroscopy) allow to solve complicated electronic structure problems.
Two examples may illustrate this approach: The neutral tris(benzene–1,2-dithiolato)chromium complex is a diamagnetic molecule with a S=0 ground state. There are two extreme electronic structures conceivable: [CrVI(bdt2-)3]0 with a central Cr(VI) ion (d0, SCr=0) and three closed-shell dianionic, diamagnetic (bdt)2-(SL=0) ligands or, alternatively the complex could possess a central paramagnetic Cr(III) ion (d3, SCr=3/2) and three paramagnetic monoanionic pi-radical ligands (bdt•)1-(SL=1/2) the spins of which couple intramolecularly antiferromagnetically yielding the observed S=0 ground state. It is possible to calculate both electronic structures: with
a) a spin-restricted closed-shell S=0 solution or
b) a broken symmetry spin unrestricted Kohn-Sham DFT solution BS(3,3).
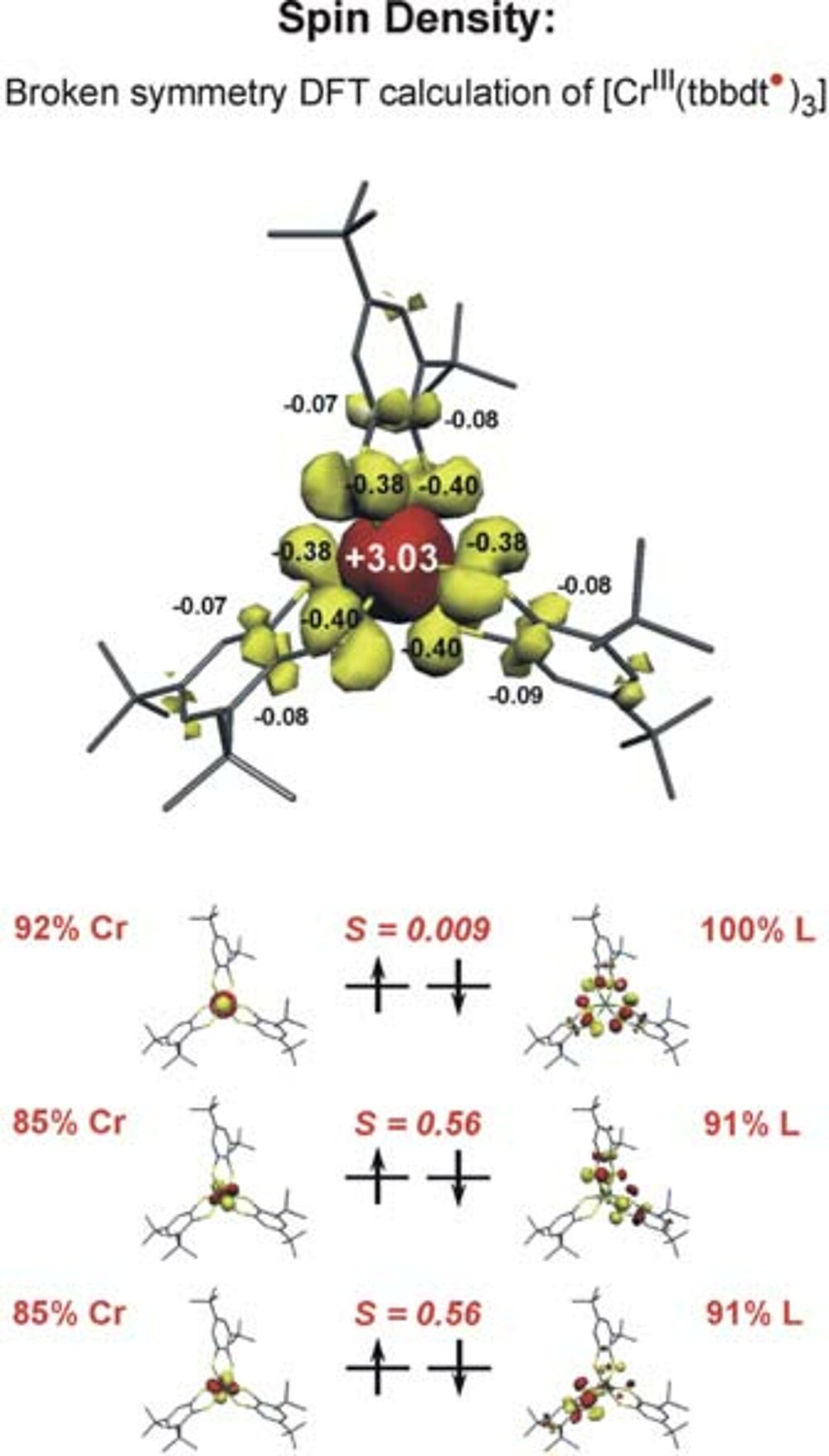
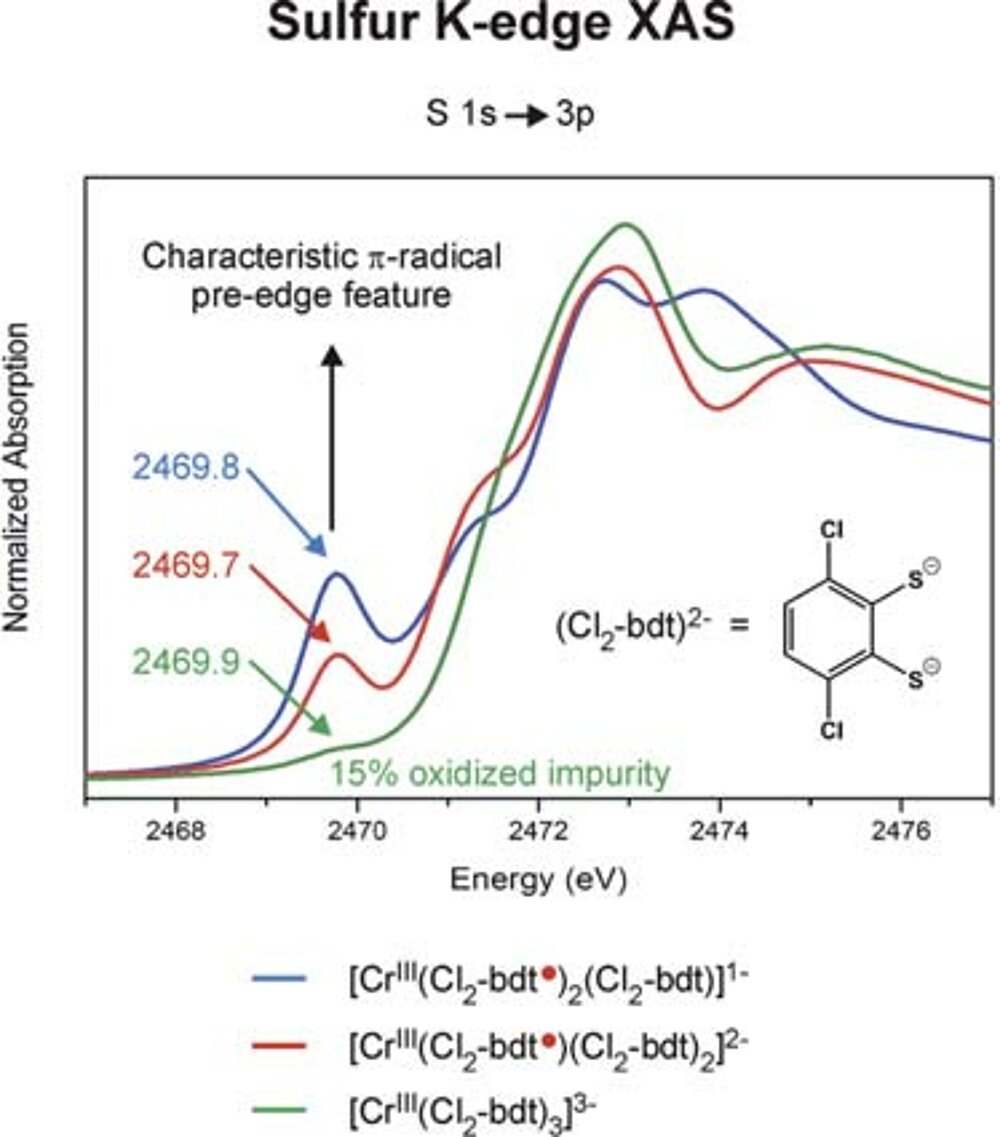
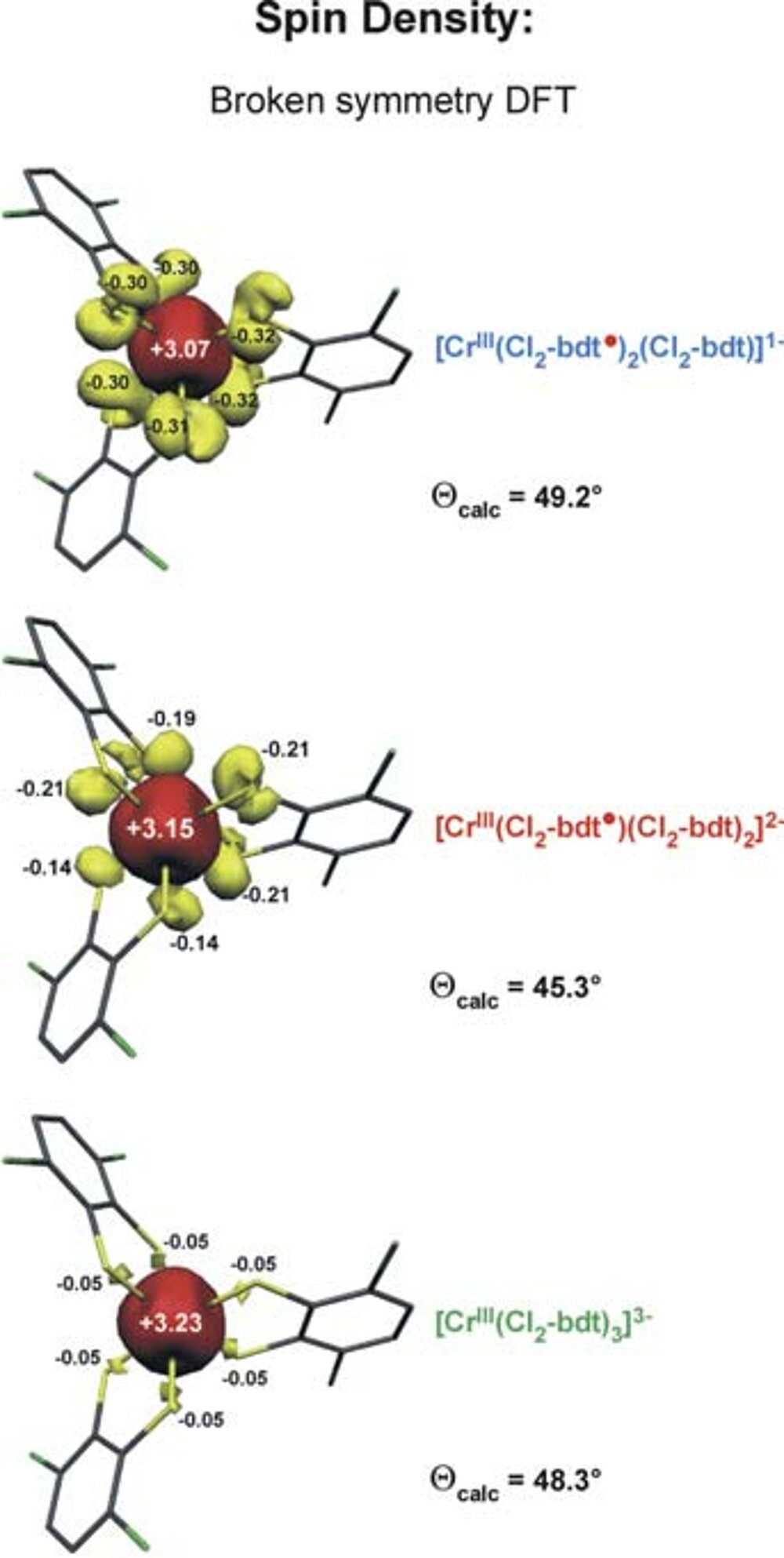
It was found that the latter solution is ~20 kcal/mol lower in energy than the former. Thus the [CrIII (bdt•) 3]0 formulation represents the correct electronic structure and not [CrVI (bdt)3]0 (Fig. 1) Experimentally this can be proven by X-ray absorption spectroscopy (measuring CrK-edge and S K-edge energies).
We have established a very close and intense collaboration between Professor Serena DeBeer George of the Stanford Synchrotron Laboratory (SLAC) and our laboratory in Mülheim. Fig. 2 shows the S K-edge spectra of the electron transfer series: [CrIII(bdt3)]3-/2-/1- where the trianion [CrIII(bdt)3]3-(S=3/2) consists of three (bdt)2-ligands whereas the dianion contains a single pi-radical anion[CrIII(bdt•)(bdt)2-]2-(S=1) and the monoanion [CrIII(bdt•)2(bdt)1-(S=1/2) consists of two (bdt•)1- radicals and one closed-shell (bdt)2-. The neutral complex consists of three (bdt•)1- monoanions and a central CrIII ion. A characteristic pi-radical pre-edge feature has been identified (of varying intensity) at 2468.8 eV for the mono- and dianion but not for the trianion. Broken symmetry DFT calculations shown in Fig. 3 beautifully confirm these results.
In an extended program we have investigated and successfully determined the electronic structures of such [ML3]n electron transfer series of V, Mo, W, Re.
A second example may illustrate the electronic structure problem in organometallic chemistry. [Ni(COD)2]0(COD=cyclooctadiene) is a diamagnetic molecule containing two neutral COD ligands and a central diamagnetic Ni0 ion(d10, SNi=0). Reaction of this species with alpha-diimines(L1) or 1,2-diketones (L2) produces diamagnetic molecules [Ni(COD) (L1,2)]0 which in the past have been described as classic Ni0 (d10) complexes with a neutral alpha-diimine or 1,2-diketone ligand.
We have established that both the alpha-diimines and the 1,2-diketones are redox-noninnocent ligands which can exist in three oxidation levels as
a) a neutral ligand (L1,2) and
b) a monoanionic pi radical (L•1,2)1-, and
c) a diamagnetic dianion (LRed)2-.
Thus the above diamagnetic complexes can have three different electronic structures:[Ni0(COD)(L1,2)]0, [NiI(COD)(L•1,2)]0, or [NiII(COD)(L1,2Red)]0.
We have established by computational chemistry and spectroscopy that they are in fact singlet diradicals with a) central NiI ion (d9, SNi=1/2) and a pi-radical monoanion(L•1,2)1- which couple antiferromagnetically: St=0. This discovery has wide implications in organometallic chemistry. In a third project – in a collaboration with Professor P.Chirik (Cornell University) and funded by the NSF (National Science Foundation, U.S.A.) and the DFG – we investigate the correlation of established electronic structures of some (bis(imino)pyridine) iron complexes and their reactivity. These ligands represent another class of redox-noninnocent ligands which can exist as a neutral species, and as mono-, di-, or trianion when coordinated to a transition metal ion.
Bioinorganic Chemistry
It is well-established that more than one half of all known enzymes are metalloproteins containing one or more transition metal ions in their respective active site (V, [Cr], Mn,Fe, Co, Ni, Cu, Zn, Mo, or W). It is our philosophy that an understanding of their reactivity can only be achieved ift he underlying coordination chemistry (geometry, electronic structure, etc.) is unraveled for all intermediates in the respective catalytic cycle.
We have continued our efforts to synthesize tetranuclear manganese complexes which might oxidize water as model compounds for the water oxidizing center (WOC) in photosystem II.
4hν + 2H2O 4H+ + 4e + O2
In this area of research a close and successful collaboration between our group and that of Professor Lubitz has been further developed. Thus, we have investigated in great depth the EPR and ENDOR signatures of MnIIIMnIVdimers in Nature (catalases, PSII) and models.
The active sites of non-heme iron metalloenzymes containing one or more iron ions continue to fascinate my group. So-called high-valent iron compounds, where the formal or spectroscopic oxidation state of the central iron ion is > +III, are still of main interest.
Vita
| Dipl. Ing.-Chem. | Eidg. Technische Hochschule (ETH), Zürich (1954) | |
| Promotion (Dr. sc. techn.) | Lab. Organische Chemie, ETH (1957) | |
| Privatdozent | Lab. Organische Chemie, ETH (1964-71) | |
| Titularprofessor | Lab. Organische Chemie, ETH (1970) | |
| Professeur ord. | Chimie Organique, Université de Genève (1971-76) | |
| Director | Max-Planck-Institut für Strahlenchemie / für Bioanorganische Chemie (1976-99) | |
| Emeritus | Max-Planck-Institut für Strahlenchemie / für Bioanorgansiche Chemie (since 1999) | |
Awards
- 1957 Silver Medal of ETH
- 1965 Swiss Chemical Society Award with Werner Medal
- 1968 Ruzicka Award, ETH Zürich
- 1990 Havinga Medal, Leiden
- 1995 M. L. Dewey and C. H. Kelly Award, University of Nebraska, Lincoln
- 1997 European Society for Photobiology Award
- 2001 Theodor Förster Award, Deutsche Bunsengesellschaft and Gesellschaft Deutscher Chemiker
Functions
- 1966-92 Visiting Professorships (University of California at Riverside and Los Angeles; Weizmann Institute of Science, Rehovot; Universities of Colorado, Boulder, Leiden, and Amsterdam; Japan Society for the Promotion of Science; Academy of Sciences, UdSSR; National Chemical Laboratory for Industry, Tsukuba, Ibaraki)
- 1970 European Photochemistry Association (Member since 1970; 1970-72 Member, Standing Committee; 1972-1976 President)
- 1971-2001 IUPAC Organic Chemistry Division (1971-81 Member, Commission on Photochemistry; 1976-81 Chairman; 1983-89 Coopted Member, Division Committee; 1991-2001 Member, Committee on Chemical Weapons Destruction Techniques)
- 1976 President, VIIth IUPAC Symposium on Photochemistry, Aix-en-Provence
- 1977 Pacific Coast Lecturer
- 1977-2000 Int. Photochemistry Foundation (1977-96 Chairman; 1996-2000 Trustee)
- 1981 Chairman, EUCHEM Conference‚ Intermediates in Organic Chemistry’, Ajaccio
- 1983-99 Founder, Biannual DFG Round Table Discussions ‘Spectroskopie biologischer Photorezeptoren’, Schloss Ringberg, Rottach-Egern
- European Society for Photobiology (Member since 1985; 1985-86 President, Founding Committee; 1986-89 Member, Executive Committee)
- Nordrhein-Westfälische Akademie der Wissenschaften, Düsseldorf (Full Member since 1986; 1998-2000 Sekretar and Vice-President)
- Since 1989 Member, Academia Europaea
- 1990-2000 Member, Beirat of the Ladislaus-Farkas Research Centre for the Conversion of Light Energy (Minerva Centre), Hebrew University, Jerusalem
- 1991 Doctor honoris causa, Institut Químic de Sarrià, Ramon Llull University, Barcelona
- Since 1993 Member, Deutsche Akademie der Naturforscher Leopoldina, Halle
- Since 1994 Member, New York Academy of Sciences
Research
Synthetic and Mechanistic photochemistry
The traditional research activities of his group – synthetic and mechanistic photochemistry – were soon complemented by a broad entry into the field of photobiology and photobiophysics. Research in photomorphogenesis and photosynthesis eventually became the predominant activities: in particular Structure, function and dynamics of chromoproteins (photoreceptors) with open-chain tetrapyrrole pigments:
- photomorphogenesis of phytochromes, the light-driven regulatory pigment in green plants, algae, moss, and cyanobacteria
- structure and energy transfer mechanism in phycobilisomes (extramembraneous light harvesting antennae of cyanobacteria and certain algae)
- photosynthesis, in particular structure/function relationships in antenna systems (LHC II, PS I-antenna complex), electron transfer processes in photosynthetic reaction centres (PS I and II), etc.



