The Challenge of Glycerol Electrooxidation
Glycerol electrooxidation (GOR) is a crucial process in the development of greener chemical synthesis technologies. The reaction involves the conversion of glycerol into valuable chemicals, such as formic acid, and other valueable 2 or 3 carbon atom containing products like oxalic, lactic or glycolic acids. However, achieving control over the selectivity of the reaction remains a significant challenge.
The Role of Electrode Geometry
In a recent study published in ChemElectroChem, researchers at the MPI CEC, led by Max Planck Research Group Leader Viktor Čolić, and their collaborators have demonstrated the significant impact of electrode geometry on the selectivity of GOR. The study used additively manufactured (AM) nickel electrodes with different geometries in hierarchical structures as electrocatalysts for GOR. The results showed that the geometry of the electrode significantly influences the selectivity of the reaction.
The Effect of Geometry on Selectivity
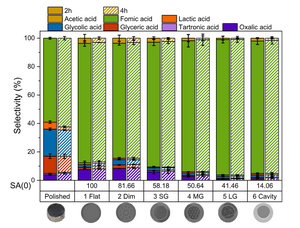
The study found that the geometry of the electrode affects the selectivity of the reaction in two ways. The results showed that an increased surface area at zero depth SA(0) correlates with higher selectivity for C2 and C3 products, as well as improved Faradaic efficiency. Secondly, the introduction of grids on the surface of the electrode enhances the nominal geometric area, while shifting the selectivity towards formic acid.
Implications of the Study
This study, a collaborative work of first author Ali Raza Khan, PhD Student at MPI CEC, and colleagues at the University of Duisburg-Essen and MPI CEC, has significant implications for the development of sustainable energy technologies. The results demonstrate that electrode geometry can be strategically tailored to optimize selectivity and enhance conversion efficiency. The very high selectivities displayed by some of the complex electrode towards formic acid are attractive, since having a reaction generating only one product can potentially enable the foregoing expensive product separation steps. On the other hand, 2- and 3-carbon atom products are economically more valuable, so the optimization of electrodes for their synthesis is interesting from another perspective.
This knowledge can be used to design more efficient electrocatalysts for GOR, which can lead to the production of valuable chemicals and fuels. The study also highlights the importance of considering macroscopic electrode geometry and structure in the design of electrocatalysts, in addition to atomic-scale properties.
Conclusion
In conclusion, the study published in ChemElectroChem demonstrated the impact of electrode geometry on the selectivity of glycerol electrooxidation. The results have important implications for the development of sustainable energy technologies and highlight the need for further research into the design of efficient electrocatalysts.
Original Paper: Khan, A.R., Kumari, B., Wegner, J., Pedrini, F., Adofo, L.A., Olean-Oliveira, A., Hagemann, U., Kleszczynski, S., Andronescu, C. and Čolić, V. (2025), Glycerol Electrooxidation at Structured Nickel Electrodes and the Effect of Geometry on the Selectivity of Product. ChemElectroChem 2500175. https://doi.org/10.1002/celc.202500175