Most studies on accelerating important electrocatalytic reactions focus on minimizing the activation energy by tuning the adsorption energies of the key intermediates. This conventional approach of increasing the reaction rate by reducing the activation energy is well established and has proven valuable in many cases.
Interestingly, however, in some cases this approach proves to be insufficient or even misleading. A team of scientists around Dr. Aleksandar R. Zeradjanin and Dr. Praveen Narangoda, research associates at MPI CEC in the Department Heterogeneous Reactions (Prof. Schlögl), has now realized that in some cases the pre-exponential frequency factor is the main determinant of the reaction rate. Modern electrocatalysis almost completely ignores this aspect.
The team has found that in some very important examples, the performance enhancement of electrocatalysts cannot be attributed to a modified/minimized activation energy.
This recent paper in ChemElectroChem therefore raises some important issues relevant to the future of electrocatalysis and electrochemical energy conversion. Particular attention is paid to the pre-exponential factor as the main determinant of electrocatalytic reaction rates.
The article also appears in 'Hot Topic: Water Splitting' and in the 'Wolfgang Schuhmann Festschrift'.
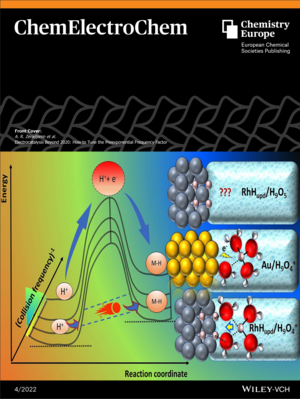
In addition, the paper is highlighted as VIP (very important paper) and listed as Front Cover.
Original Publication: Narangoda, P., Spanos, I., Masa, J., Schlögl, R. and Zeradjanin, A.R. (2022), Electrocatalysis Beyond 2020: How to Tune the Preexponential Frequency Factor. ChemElectroChem. https://doi.org/10.1002/celc.202101278
The Front Cover shows how metals with more destabilized interfacial water molecules could behave as efficient electrocatalysts for hydrogen evolution reaction (HER) where high reaction rate, despite of relatively high activation energy, is possible to achieve via high values of preexponential factor by proton tunnelling. While destabilization of the proton’s solvation sphere in acidic electrolytes is intuitive, in alkaline media this proceeds via a more complex mechanism. More information can be found in the Article by P. Narangoda et al.