Long-chain alcohols - what's behind them?
Long-chain alcohols are chemical compounds that play a central role in our everyday lives. Among other things, they are used in the production of surfactants for cleaning agents, in lubricants, plasticisers, personal care products and even in more environmentally friendly fuels. Due to their wide range of applications, they are an indispensable component of modern industrial and consumer products.
Methanol as a key raw material for the future
Methanol, the smallest alcohol molecule, is also an important energy and hydrogen carrier molecule and is becoming increasingly important in the transition from fossil to renewable raw materials, as it can be produced from CO₂ and renewable H2. In places with a surplus of renewable energy - such as solar or wind farms - methanol can be produced cost-effectively in large quantities. It can then be easily transported and used as a versatile raw material at other locations: for energy generation, as a building block for chemical processes or for the production of hydrogen. The Department of Molecular Catalysis (Prof Walter Leitner) has also previously shown how methanol can be used as a synthesis gas (CO and H2) surrogate through dehydrogenation (https://doi.org/10.1002/anie.202110910).
A groundbreaking method for the production of long-chain alcohols
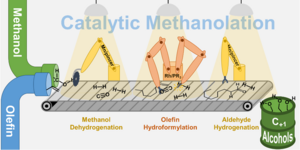
Researchers from the Multiphase Catalysis team have developed an innovative method to convert methanol together with olefins (a group of chemical compounds consisting of hydrocarbons) into long-chain alcohols. Olefins are a common raw material in the chemical industry. The method works via a so-called tandem catalytic reaction system. This means that several chemical reactions take place in a kind of ‘reaction chain’ that interlock seamlessly.
Methanol is first converted into synthesis gas, which is then brought together with olefins to form long-chain alcohols. This process uses two specialized catalysts - substances that accelerate chemical reactions without being consumed themselves. The catalysts used consist of manganese and rhodium complexes that have been chemically customized to make the reactions particularly efficient.
More precisely:
The multiphase catalysis team has developed a tandem reaction system that consists of three individual steps (so-called ‘methanolation’). The dehydrogenation of methanol to synthesis gas is combined with the hydroformylation and hydrogenation of olefins to C+1 alcohols. In this way, methanol is added to the olefin in a 100% atom-efficient manner. The reaction system combines two homogeneous organometallic catalysts, a pincer-modified manganese complex and a rhodium complex modified with simple phosphine ligands.
Why is this a major step forward?
The new method is particularly resource-saving and environmentally friendly. Here are some reasons why:
- cost & energy efficient: the process does not require high pressure gas infrastructure or specialized equipment, making it cost-effective, safer and more accessible on a laboratory and industrial scale.
- sustainable: methanol, a climate-friendly raw material, is fully incorporated into the end product without generating waste.
- precise: the system produces preferentially linear alcohols - a form that is particularly useful and valuable in industry.
The importance for a sustainable future
This method could bring the chemical industry one step closer to the vision of a carbon-neutral economy. It shows how green energy and sustainable raw materials can be integrated into efficient production processes. The project is an example of how scientific innovation can help replace fossil resources and create new ways to manufacture everyday products.
The researchers hope that their work will inspire other scientists to develop similar approaches that accelerate the transition to a carbon-neutral industry and make the goal of net-zero carbon emissions more achievable.
Associated Publication:
Sebastian Stahl, Jeroen T. Vossen, Stephan Popp, Walter Leitner, Andreas J. Vorholt, “Methanolation of Olefins: Low-Pressure Synthesis of Alcohols by the Formal Addition of Methanol to Olefins” in Angew. Chem. Int. Ed. 2024, e202418984. https://doi.org/10.1002/anie.202418984