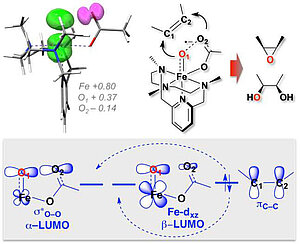
Scientists from the Max Planck Institute for Chemical Energy Conversion in Mülheim an der Ruhr revealed the “true” oxidant in the Fe(II)-catalyzed selective C=C oxidation as a [FeIV(O)(OC(O)R)2–]2+, with an iron(IV) centre antiferromagnetically coupled to an O-O radical, that was further characterized as a half-bond O O species. This insight was possible to make based on the correlation of the electronic structure calculations of oxo-iron species with the experimental spectroscopic properties (EPR, Mössbauer, resonance Raman) and reactivity.
This finding could answer the long-standing questions regarding the stereo-specifity observed in iron (II)-based catalytic systems. Moreover, this work provides important progress in understanding the role of oxo-iron species in the catalytic cycle of Rieske dihydroxylating enzymes.
These results have just been published in the Journal of the American Chemical Society: Mondal, B., Neese, F., Bill, E., Ye, S. Electronic Structure Contributions of Non-Heme Oxo(V) Complexes to the Reactivity <link https: doi.org jacs.8b04275 _blank external-link-new-window internal link in current>