The soluble methane monooxygenase (sMMO) is a multiprotein complex and one of two enzymes in nature capable of converting methane into methanol. Therefore, the protein and its capabilities are of great interest for fuel conversion technology.
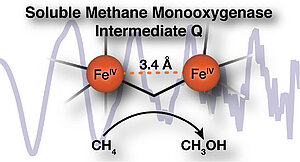
The Q intermediate of methane monooxygenase is particularly important and interesting in the conversion process. Q has the unique ability to oxidize the very strong C-H bond of methane and then introduce an oxygen atom to form methanol. This very basic chemical reaction is not yet well characterized.
A team of scientists led by <link internal-link internal link in current>Prof. Serena DeBeer, director of the department '<link internal-link internal link in current>Inorganic Spectroscopy' at the MPI CEC, hopes to gain a better understanding of the structure of the Q intermediate and has used X-ray absorption spectroscopy to look more closely at the structure and, in particular, the distance between two iron atoms. The exact naming of the distance can help to determine whether Q has a "diamond" diiron core structure or an open core structure.
Previous EXAFS analyses showed that the two irons of the active site were very close to each other (2.46 Å). Using high-resolution EXAFS spectroscopy, the scientists were now able to determine, without influencing background signals, that there is no evidence of a short Fe-Fe distance, but rather a long 3.4 Å diiron distance, as observed in open synthetic model complexes.
This finding, published in November in <link https: pubs.acs.org doi jacs.8b10313 external-link-new-window internal link in current>JACS, contributes significantly to the understanding of biological methanol production. In addition, Prof. DeBeer's research shows that unwanted background signals contaminated previous data and thus misled analyses. New, ultra-modern EXAFS spectroscopy makes it possible to suppress the interfering background signals. This has broad implications for EXAFS studies on all diluted ferrous samples.
Publication: Cutsail III, G.E., Banerjee, R., Zhou, A., Que, L., Lipscomb, J.D., DeBeer, S. (2018). High-Resolution EXAFS Provides Evidence for a Longer Fe•••Fe Distance in the Q Intermediate of Methane Monooxygenase Journal of American Chemical Society <link https: doi.org jacs.8b10313 external-link-new-window internal link in current>