The electrochemical reduction of nitro substrates is emerging as a powerful and sustainable approach in organic synthesis. A recent review published in ACS Electrochemistry by Sebastián O. Simonetti, Sebastian B. Beil, and Siegfried R. Waldvogel surveys the transformative potential of this method. Titled Nitro Substrates in Reductive Electrosynthesis: A Review, the paper highlights how electroreduction processes enable the selective conversion of nitro compounds into valuable nitrogen-containing products without the need for metal-based reducing agents. This method represents a significant step forward in sustainable chemistry, aligning with green chemistry principles by minimizing waste and hazardous reagents.
Harnessing Electrochemistry for Selective Reductions
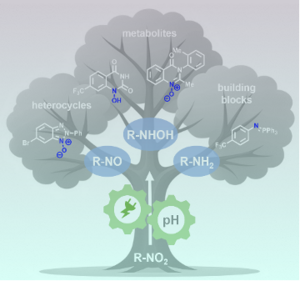
One of the key advantages of electrochemical reduction is its precise tunability. By simply adjusting the pH of the electrolyte and current density, researchers can control the degree of reduction of nitro groups, allowing for the efficient production of hydroxylamines, N-hydroxyl, and N-oxy derivatives - compounds traditionally challenging to synthesize by conventional methods. The in-situ formation of reactive nitrogen intermediates further broadens the synthetic scope, making it possible to access a variety of nitrogen-based products through follow-up condensation or substitution reactions. This electro-organic approach not only increases the sustainability of the synthesis but also improves scalability and safety compared to conventional reduction methods.
A Pathway to Greener Synthesis
Electrosynthesis stands out for its environmental benefits. Unlike traditional methods that rely on precious metals and produce considerable waste, electrochemical reduction uses electric current and protic media as the sole reducing agent. This current can be sustainably sourced from renewable energy like photovoltaics or wind power, significantly reducing the carbon footprint of chemical manufacturing. Additionally, the galvanostatic operation in electrosynthesis optimally balances interactions between electrodes and electrolytes, enhancing both scalability and safety. These innovations contribute to the broader movement towards greener and more sustainable chemical processes in both academic and industrial settings.
Leading Authors in Sustainable Chemistry
The review was authored by Dr. Sebastián O. Simonetti, postdoc at the MPI CEC, Dr. Sebastian Beil, head of the group “Electrosynthesis and Photocatalysis” and Prof. Siegfried R. Waldvogel, director of the department “Electrosynthesis”. Their collaborative research focuses on advancing green chemistry through electrochemical methods. Their expertise in electro-organic synthesis and sustainable reaction design is well-recognized in the field, with a commitment to reducing environmental impact while enhancing synthetic efficiency. Their latest work provides valuable insights into how electrosynthesis can redefine sustainable chemical manufacturing. A corresponding illustration of the review was also featured as supplementary cover art of the journal ACS Electrochemistry.
With their findings, Simonetti, Beil, and Waldvogel have not only mapped out the current landscape of nitro substrate electroreduction but also paved the way for future advancements in sustainable organic synthesis. Their review underscores the promise of electrochemical methods in building a more sustainable chemical industry.
Original Paper: Nitro Substrates in Reductive Electrosynthesis: A Review
Sebastián O. Simonetti, Sebastian B. Beil, and Siegfried R. Waldvogel
ACS Electrochemistry Article ASAP
DOI: 10.1021/acselectrochem.5c00081

![[Translate to EN:]](/fileadmin/_processed_/c/8/csm_240605_Cover_aeclc7.2025.1.issue-6.xlargecover-2_6e63ff15c3.jpg)