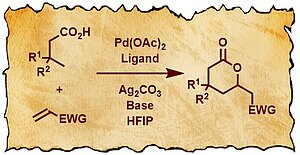
Carboxylic acid groups are contained in small organic molecules used for many different applications, for example as pharmaceuticals, flavors or odorants. One way of constructing such molecules from simpler precursors is via a C–H activation. Whereas there have been examples in the literature for such transformations using various carboxylic acid derivatives, the employment of free carboxylic acids for this purpose has remained highly challenging.
In a recent study published in Angewandte Chemie International Editon, the van Gemmeren research group (Otto Hahn Research Group) has developed a novel catalyst system that is able to overcome these challenges and effect a highly efficient C–H activation in the remote γ-position. A subsequent intramolecular Michael addition then produces valuable δ-lactones in a straightforward manner. In addition to this discovery, detailed mechanistic studies were conducted and delivered valuable insights into the nature of the catalytic cycle.
Original publication: Ghosh, K.K., Uttry, A., Mondal, A., Ghiringhelli, F., Wedi, P., van Gemmeren, M. (2020). Ligand-Enabled γ-C(sp3)–H Olefination of Free Carboxylic Acids Angewandte Chemie International Edition <link https: doi.org anie.202002362>doi.org/10.1002/anie.202002362