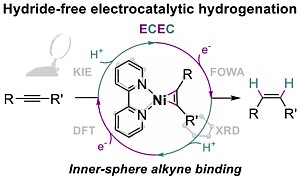
The OMeCat team led by Nicolas Kaeffer in the Molecular Catalysis Department of Prof. Leitner has brought out that the molecular electrocatalytic version of alkyne semihydrogenation can bypass hydride intermediates, otherwise key in classical hydrogenation.
Through a combined experimental and theoretical study, the post-doctoral researcher Gabriel Durin and colleagues disclosed that hydrogenation proceeds there via a sequence of electron and proton transfer steps in an inner-sphere fashion that involves alkyne binding to the reduced nickel catalyst.
This approach, so far barely documented for the reduction of organic unsaturated compounds, may open up new paradigms for selective hydrogenation or hydroelementation reactions, particularly in redox, photocatalytic or electrocatalytic systems.
Original Publication:
Gabriel Durin, Mi-Young Lee, Martina A. Pogany, Thomas Weyhermüller, Nicolas Kaeffer, and Walter Leitner (2023) Hydride-Free Hydrogenation: Unraveling the Mechanism of Electrocatalytic Alkyne Semihydrogenation by Nickel–Bipyridine Complexes. Journal of the American Chemical Society, Article ASAP DOI: 10.1021/jacs.3c03340