High-valent iron complexes with coordinated O2– or N3– ligands are key intermediates in the chemical activation of O2 and N2 molecules. At room temperature, these small molecules are sluggish reactants and their activation for instance for application in fuel cells requires efficient catalysts.
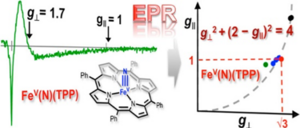
Synthetic iron nitride complexes (with a N3- ligand) allow scientists to study the most critical step for an important activation pathway in detail. In particular, scientists of the Max Planck Institutes in Mülheim could uncover the electronic structure of the first ever described iron(V) nitride complex, with a porphyrin as supporting ligand, to be a genuine very high-valent iron(V) system with orbitals near degenerate S = 1/2 ground state.
This was achieved by using complementary Electron Paramagnetic Resonance (EPR) and 57Fe Mössbauer spectroscopic measurements and thorough interpretation of closely related, spectroscopy-based quantum-chemical calculations (wavefunction-based CASSCF methods). As a general outcome, the scientists could establish a systematic relation between EPR g-tensor components, g|| and g⊥ for the iron(V)-nitrido and -oxo complexes in tetragonal supporting ligand environments.
This theoretical rule should aid in detecting other, usually short-lived, high-valent iron intermediates and understanding their functions in complex biological or industrial processes.
The research that led to this finding was carried out in a collaboration between teams from both Max Planck Institutes in Mülheim an der Ruhr (Joint Work Space), headed by Dr. Eckhard Bill (<link https: www.cec.mpg.de external-link-new-window internal link in current>MPI für chemische Energiekonversion) and Dr. Shengfa Ye (<link https: www.kofo.mpg.de external-link-new-window internal link in current>MPI Kohlenforschung). These results have recently been published in the <link https: pubs.acs.org doi jacs.8b11429 external-link-new-window internal link in current>Journal of the American Chemical Society.
Publication: Chang, H-C., Mondal, B., Fang, H., Neese, F., Bill, E., Ye, S. (2019). EPR Signature of Tetragonal Low Spin Iron(V)-Nitrido and -Oxo Complexes Derived from the Electronic Structure Analysis of Heme and Non-Heme Archetypes. Journal of American Chemical Society 141(6), 2421-2434. <link https: doi.org jacs.8b11429 external-link-new-window internal link in current>