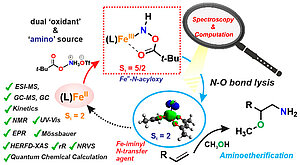
A collaborative effort from the experimental team of scientists led by Prof. Serena DeBeer from MPI CEC and theoretical chemists from Prof. Frank Neese’s team at MPI für Kohlenforschung deciphers the mechanism of a complex, iron-catalyzed amination at the level of electronic structure. Amination is the process by which an amine group is introduced into an organic molecule.
The focus of the work shows how important a close link between experiment and theory is for a complete understanding of the reaction paradigm and provides a perfect example of the successful collaboration between the two departments, Inorganic Spectroscopy and Molecular Theory and Spectroscopy at the Mülheim Chemistry Campus.
Amines are found ubiquitously throughout the natural world as key functional groups in amino acids and nucleotide bases, and are fundamental components of pharmaceuticals, agrochemicals, dyes and polymers. To date, deciphering "amino functionality" remains one of the greatest challenges in organic synthesis. An attractive approach to address this challenge is the direct catalytic amination of organic molecules. Iron-catalyzed direct synthesis of unprotected amines using N-O reagents derived from hydroxylamines as the amine source has been extensively studied by the catalytic community using synthetic iron catalysts as well as artificially prepared iron proteins. However, the mechanism underlying these amination reactions and nature of intermediates involved remained elusive.
In this work, recently published in JACS, a variety of analytical and spectroscopic techniques were used to understand the geometrical and electronic structure of the reaction components involved and the mechanism of the iron-catalyzed amino functionalization reaction. The results obtained from the experimental techniques were correlated with computational protocols to enable a clear understanding of the catalytic reaction mechanism and the contribution of the reactive intermediates to N-group transfer activity.
The insights obtained in this work, regarding the electronic structures and reaction mechanism from a combination of experimental and theoretical studies, would shed light on the design of new, improved catalysts for group transfer chemistry. This comprehensive team work is expected to have wider implications in correlating the field of catalysis and reaction design to spectroscopy and theory
Lead authors in this study: Dr. Sayanti Chatterjee, Prof. Serena DeBeer, Prof. Frank Neese, Ingolf Harden
Original Publication: S. Chatterjee, I. Harden, G. Bistoni, R. G. Castillo, S. Chabbra, M. van Gastel, A. Schnegg, E. Bill, J. A. Birrell, B. Morandi, F. Neese, S. DeBeer (2022) A Combined Spectroscopic and Computational Study on the Mechanism of Iron-Catalyzed Aminofunctionalization of Olefins Using Hydroxylamine Derived N–O Reagent as the “Amino” Source and “Oxidant”. J. Am. Chem. Soc. 2022, 144, 6, 2637–2656. https://doi.org/10.1021/jacs.1c11083