Catalysis is a fundamental pillar of the chemical industry and is used across the whole value chain ranging from the production of fuels, fine chemicals, agrochemicals, and pharmaceuticals. With the rise of alternative renewable energy sources, the permanent evolution of catalysts and catalytic processes is crucial to face the inevitable variations in electricity production. In particular, energy efficiency, benignity of reaction conditions, and adaptivity to intermittent energy supply are parameters of ever increasing importance. In this context, magnetic induction offers the possibility to heat directly magnetic nanocatalysts in an extremely localized, rapid, and energy efficient manner, saving the need to heat the complete reactor environment (solvent, reactor parts, etc.). In turn, this results in milder overall process temperatures and lower energy consumption. In addition, the rapid heating and cooling of magnetically heated nanocatalysts is of great interest to address the challenges associated to the use of fluctuating renewable energy. While direct catalyst heating via magnetic induction offers promising new perspectives for process intensification, the development of catalytic systems capable of reaching high temperatures under magnetic induction activation is particularly difficult. As a result, the benefits of magnetic induction are until now only accessible using complex and specifically designed materials.
To tackle this challenge, a team of scientists lead by Dr. Alexis Bordet, responsible for the “Multifunctional Catalytic Systems” team in the department of Prof. Walter Leitner at the MPI CEC, now reports an innovative strategy to design, synthesize, and apply catalytic systems activated by magnetic induction heating. By combining expertise on magnetic nanoparticles and nanoparticle-based catalysis (“Multifunctional Catalytic Systems” team, MPI CEC) as well as chemical engineering (“Multiphase Catalysis” team lead by Dr. Andreas J. Vorholt, MPI CEC), the interdisciplinary research team managed for the first time to bestow a commercial heterogeneous catalyst with magnetic heating capabilities. The resulting multifunctional catalytic system was applied to a continuously operated liquid-phase hydrogenation reaction, showing outstanding stability and tolerance to intermittent power supply.
The study recently published in Angewandte Chemie International Edition demonstrates the possibility to modify even standard heterogeneous catalysts with novel physical properties in a simple and versatile manner. This strategy should be transferrable readily to a wide range of heterogeneous catalysts and transformations, thus paving the way toward the development (or modification) of catalytic systems and processes that can meet the current societal expectations in terms of energy efficiency and use of fluctuating renewable energy.
Scientific details:
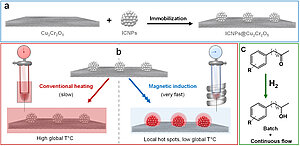
The scientists have prepared a multifunctional hydrogenation catalyst composed of commercial Cu2Cr2O5 decorated with ferromagnetic iron carbide nanoparticles possessing excellent induction heating performance (commonly defined by the Specific Absorption Rate, SAR of the receiving material). The resulting ICNPs@Cu2Cr2O5 multifunctional catalytic system was applied to the selective hydrogenation of various aromatic ketones to aromatic alcohols, a transformation that plays an important role for the conversion of biogenic feedstock as well as for the production of fine chemicals and pharmaceuticals. Activated by magnetic induction, ICNPs@Cu2Cr2O5 effectively hydrogenated a scope of benzylic and non-benzylic ketones under mild conditions (~ 80°C, 3 bar H2, heptane) thanks to local hot spots generated by the ICNPs at the Cu2Cr2O5 surface. In contrast, ICNPs@Cu2Cr2O5 and Cu2Cr2O5 were found inactive using conventional heating under similar conditions. Recycling and long-term experiments conducted under batch (10 cycles) and continuous flow conditions (17 h on stream) revealed the excellent reusability and high long-term stability of the ICNPs@Cu2Cr2O5 catalyst.
Future studies will aim at extending this concept and at exploring the potential of magnetically induced local heating for the development of multifunctional catalytic systems with switchable and adaptive reactivity.
Original paper: H. Kreissl, J. Jin, S.-H. Lin, D. Schüette, S. Störtte, N. Levin, B. Chaudret, A. J. Vorholt, A. Bordet, W. Leitner, Commercial Cu2Cr2O5 Decorated with Iron Carbide Nanoparticles as a Multifunctional Catalyst for Magnetically Induced Continuous-Flow Hydrogenation of Aromatic Ketones. Angew. Chem. Int. Ed. 2021, 60, 2–10. https://doi.org/10.1002/anie.202107916
For further information on ICNPs and magnetic induction heating: A. Bordet, L.-M. Lacroix, P.-F. Fazzini, J. Carrey, K. Soulantica, B. Chaudret, Magnetically Induced Continuous CO2 Hydrogenation Using Composite Iron Carbide Nanoparticles of Exceptionally High Heating Power. Angew. Chem. Int. Ed. 2016, 55, 15894-15898. https://doi.org/10.1002/ange.201609477
Funding acknowledgement
- Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany ́s Excellence Strategy – Exzellenzcluster 2186 ‘The Fuel Science Center’ (ID: 390919832)
- Max-Planck-Gesellschaft (Max Planck Society)