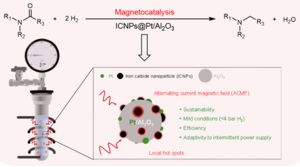
The transformation of amides into amines is an important process in the production of pharmaceuticals and fine chemicals. However, this reaction has long presented a major challenge due to the stability of the carbon-oxygen (C=O) bond in amides. Although using hydrogen gas (H₂) for this transformation is considered a green and sustainable approach, it often requires high pressures and specialized equipment, limiting its practical use.
Recognizing the potential of this transformation, the ACS Green Chemistry Institute Pharmaceutical Roundtable (GCIPR) has identified amide hydrogenation as one of the most desirable processes for advancing sustainable chemical synthesis. Making this reaction more accessible and efficient has therefore been a long-standing goal in both academic and industrial research.
Now, researchers at the MPI CEC have found a novel solution. In a new study published in Nature Communications, the team led by Dr. Alexis Bordet - within the Department of Molecular Catalysis headed by Prof. Walter Leitner - demonstrates that magnetocatalysis can make this challenging reaction not only possible, but efficient under much milder conditions.
A key role in this discovery was played by Dr. Sheng-Hsiang Lin and Sihana Ahmedi, who share first authorship of the publication. Dr. Lin, now an alumnus of the institute, and Sihana Ahmedi, a current PhD student in the Bordet team, led the experimental work and played central roles in the design, implementation, and interpretation of the study. Their contributions were essential in demonstrating the effectiveness of magnetic induction as a tool to enable hydrogenation under mild conditions - paving the way for further innovation in adaptive and sustainable catalysis.
The approach, known as magnetically-induced catalysis, uses iron carbide nanoparticles (ICNPs) placed alongside a standard platinum-based catalyst. When exposed to an alternating magnetic field, these nanoparticles generate localized heat precisely where it is needed. This targeted energy helps activate the platinum catalyst, enabling the hydrogenation of a wide variety of amides even at ambient hydrogen pressures. Impressively, the experiments were carried out using regular glassware rather than specialized high-pressure reactors.
The new catalytic system also showed resilience under variable electricity supply, suggesting it could be powered by intermittent renewable energy sources like solar or wind power - further enhancing its sustainability.
This work opens new possibilities not only for greener amide-to-amine conversions, but also for applying magnetic induction to other reactions that currently require harsh conditions. By combining adaptability, efficiency, and environmental responsibility, this innovation marks a significant step forward in the field of sustainable chemical synthesis.
Original Paper:
- S.-H. Lin, S. Ahmedi, A. Kretschmer, C. Campalani, Y. Kayser, L. Kang, S. DeBeer, W. Leitner, A. Bordet. Low Pressure Amide Hydrogenation Enabled by Magnetocatalysis. Nat. Comm. 2025, 16, 3464. https://doi.org/10.1038/s41467-025-58713-6
For further information on adaptive catalytic systems and the use of magnetic induction in catalysis:
- Adaptive Catalytic Systems for Chemical Energy Conversion. A. Bordet, W. Leitner, Angew. Chem. Int. Ed. 2023, 62, e202301956. https://doi.org/10.1002/anie.202301956