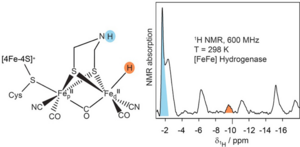
The [FeFe] hydrogenases are known so far as the most efficient catalysts for H2 production. In the current working model of this process, the hydride state intermediate has been recognized as a crucial step during the H2 conversion. However, the occurrence of this intermediate has remained without direct experimental evidence under (nearly) physiological conditions. Scientists from the Max Planck Institute for Chemical Energy Conversion in collaboration with the NMR group from the neighbouring Max Planck Institute for Coal Research in Mülheim an der Ruhr, have provided a direct evidence of a hydride intermediate of the [FeFe] hydrogenase at ambient conditions.
1H NMR experiments were performed on maturated model complexes of the intermediate state, in either unlabelled or/and deuterated form as well as on wildtype [FeFe] hydrogenase. A clear signal at about -9.6 ppm was observed in the spectra of the samples not being fully deuterated, and together with the spectra comparison of the model complexes set was assigned to a solvent exchangeable 1H (terminal hydride).
In this study, for the first time, the possibility of using NMR spectroscopy to detect a labile hydride intermediate of an enzyme in solution at room temperature was shown. This opens up a new perspective to study catalytic mechanism of i.e. [FeFe] hydrogenase.
This collaborative work from the researches of the Mülheim Chemistry Campus was recently published in <link https: pubs.acs.org doi abs jacs.8b00459 external-link-new-window internal link in current>Journal of American Chemical Society.