Dr. Sergey Peredkov - X-ray Spectroscopy Instrumentation
- Dr. Sergey Peredkov
- Group leader
- X-ray Spectroscopy Instrumentation
- Inorganic Spectroscopy
- +49 (0)30 / 8062-14807
- sergey.peredkov(at)cec.mpg.de
Vita
| Diploma (Engineer-Physicist) | National Research Nuclear University, The Faculty of Experimental and Theoretical Physics, Moscow, Russia (1993) |
| Research-engineer | Kurchatov Synchrotron Radiation Source, Moscow, Russia (1993-1998) |
| Research-engineer | MAX-lab, Lund University, Sweden (1998-2003) |
| Ph.d. (Physics) | Lund University, Natural Science Faculty, Department of Synchrotron Radiation Research, Sweden (2007) |
| Postdoc | BESSY II synchrotron source, Berlin, Germany (2007-2011) |
| Support Scientist | MAX-lab, Lund University, Sweden (2011-2013) |
| Scientist | MPI CEC, Department of Molecular Theory and Spectroscopy (2013-2016) |
| Group leader | MPI CEC, Department Inorganic Spectroscopy (since 2017) |
Publications
Full publications list | ResearcherID
Selected MPI CEC publications
- Liu, Y., Chatterjee, S., Cutsail III, G. E., Peredkov, S., Gupta, S. K., Dechert, S., DeBeer, S., Meyer, F. (2023). Cu4S Cluster in "0-Hole" and "1-Hole" States: Geometric and Electronic Structure Variations for the Active Cu-Z* Site of N2O Reductase. Journal of the American Chemical Society, 145(33), 18477-18486. doi:10.1021/jacs.3c04893.
- Geoghegan, B. L., Liu, Y., Peredkov, S., Dechert, S., Meyer, F., DeBeer, S., Cutsail III, G. E. (2022). Combining Valence-to-Core X-ray Emission and Cu K-edge X-ray Absorption Spectroscopies to Experimentally Assess Oxidation State in Organometallic Cu(I)/(II)/(III) Complexes. Journal of the American Chemical Society, 144(6), 2520-2534. doi:10.1021/jacs.1c09505.
- Zimmermann, P., Peredkov, S., Abdala P.M., DeBeer, S., Tromp, M., Müller, C., van Bokhoven, J.A. (2020). Modern X-ray spectroscopy: XAS and XES in the laboratory Coordination Chemistry Reviews 423, 213466. https://doi.org/10.1016/j.ccr.2020.213466
- Levin, N., Peredkov, S., Weyhermüller, T., Rüdiger, O., Pereira, N.B., Grötzsch, D., Kalinko, A., DeBeer, S. (2020). Ruthenium 4d-to-2p X-ray Emission Spectroscopy: A Simultaneous Probe of the Metal and the Bound Ligands Inorganic Chemistry 59(12), 8272-8283. https://doi.org/10.1021/acs.inorgchem.0c00663
- Szlachetko J., Nachtegaal M., Grolimund D., Knopp G., Peredkov S., Czapla–Masztafiak J., Milne C.J. (2017). A Dispersive Inelastic X-ray Scattering Spectrometer for Use at X-ray Free Electron Lasers Applied Sciences 7(9), 899. https://doi.org/10.3390/app7090899
- Peredkov S., Peters S., Al-Hada M., Erko A., Neeb M., Eberhardt W. (2016). Structural investigation of supported Cun clusters under vacuum and ambient air conditions using EXAFS spectroscopy
Catalysis Science & Technology 6(18), 6942-6952. https://doi.org/10.1039/c6cy00436a - Meyer J., Niedner-Schatteburg G., Peredkov S., Eberhardt W., Neeb M., Palutke S., Martins M., Wurth W. (2015). The spin and orbital contributions to the total magnetic moments of free Fe, Co and Ni clusters Journal of Chemical Physics 143(10), 104302. https://doi.org/10.1063/1.4929482
- Al-Hada, M., Peters, S., Peredkov, S., Neeb, M., Eberhardt, W. (2015) Nanoisland formation of small Agn-clusters on HOPG as determined by inner-shell photoionization spectroscopy Surface Science 639, 43-47. https://doi.org/10.1016/j.susc.2015.03.016
- Dielman, D., Tombers, M., Peters, L., Meyer, J., Peredkov, S., Jalink. L., Neeb, M., Eberhardt, W., Rasing, T., Niedner-Schstteburg, G., Kirilyuk, A. (2015). Orbit and spin resolved magnetic properties of size selected [ConRh]+ and [ConAu]+ nanoalloy clusters Physical Chemistry Chemical Physics 17(41), 28372-28378. https://doi.org/10.1039/c5cp01923k
Awards
X-ray Spectroscopy Instrumentation
The PINK project is devoted to establishing a high-resolution (∆E=0.2-0.7 eV) X-ray emission spectroscopy (XES) setup at the BESSY II synchrotron ring in Berlin, opening up “two-color” and time-resolved studies of catalytic systems at an unprecedented level. From natural systems to manmade homogenous and heterogeneous catalysts, transition metals are often employed to effect challenging chemical transformations. Knowledge of the changes in the transition metal electronic structure and ligation environment are essential for any detailed mechanistic understanding, which forms the basis for rational catalysis design.
Our understanding of the electronic structure of matter is largely based on studies of the interaction of matter with electromagnetic radiation or with particles and on the concurrent development of quantum-mechanical models. To this end, XES is a powerful element selective technique, which provides local structural information near the selected atomic species [1]. XES measurements and analysis of x-ray emission lines can reveal the ligand identity, the ligand ionization potential and the metal-ligand distance. XES can also help to identify oxidation states of the extent of substrate activation [2,3,4,5]. In contrast to photoelectron spectroscopy (PES), XES is a photon-in-photon-out technique, thus the probing depth is roughly two orders of magnitude larger than in PES. XES is a "photon-hungry" technique and synchrotron radiation is the optimal source of x-ray radiation for studies of dilute systems.
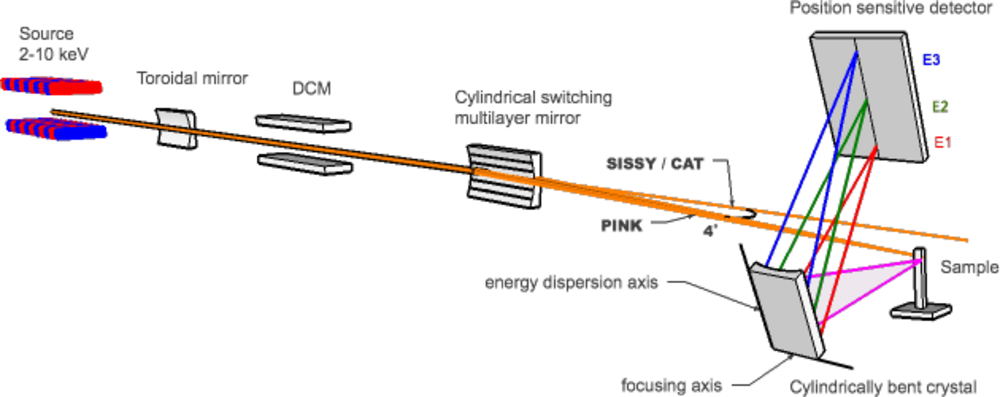
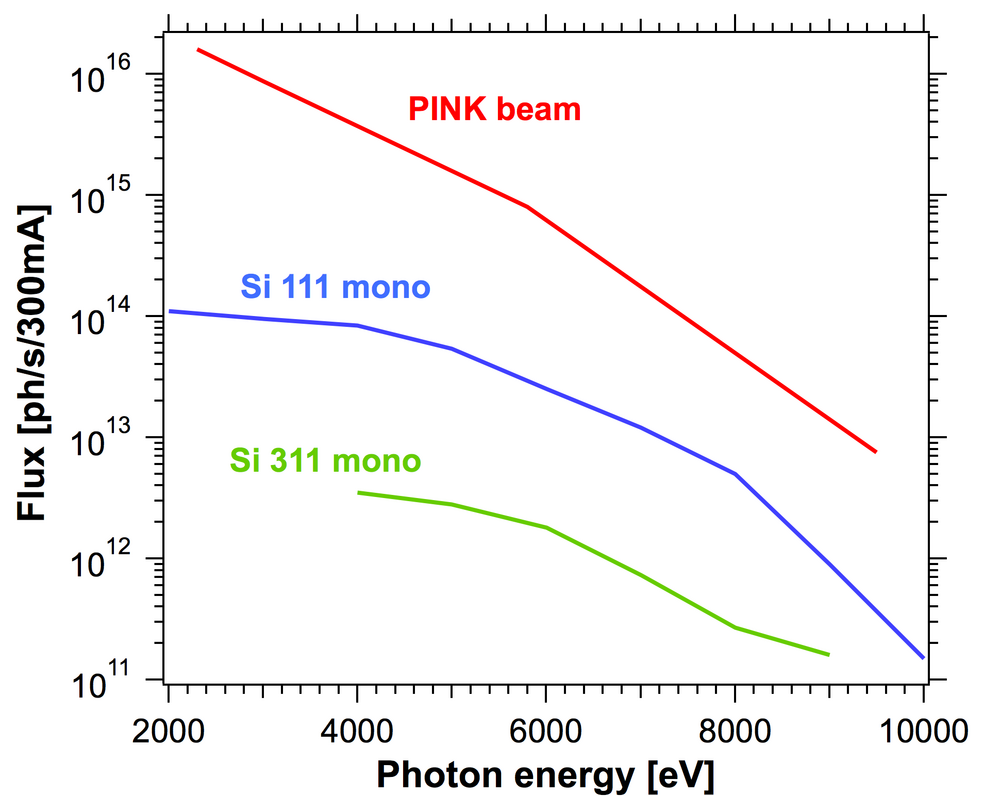
The PINK setup is designed to operate in the tender x-rays regime with energies ranging from 2 to 10 keV (Fig. 1). This unique energy range provides access to both non-resonant and resonant XES studies of transition metals ranging from Ti to Cu (Kα, Kβ lines) and Zr to Ag (Lα, Lβ lines), as well as lighter elements including P, S, Cl, K, Ca (Kα, Kβ lines). The optical system of the beamline has two modes of operation: a pink mode and a monochromatic mode. A high intensity undulator source combined with a multilayer monochromator delivers the high photon flux at the sample allowing non-resonant XES measurement of very dilute substances (Fig. 2).
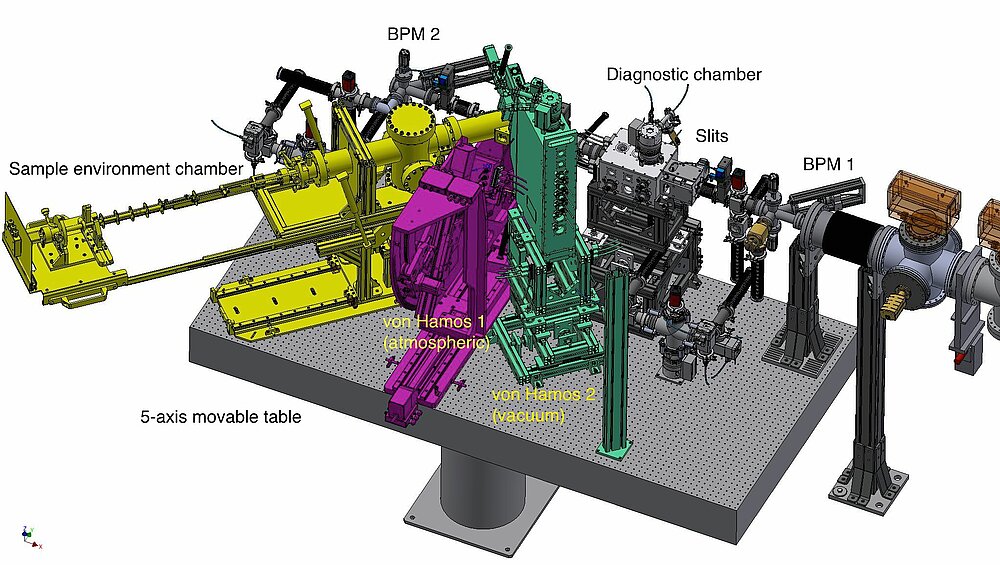
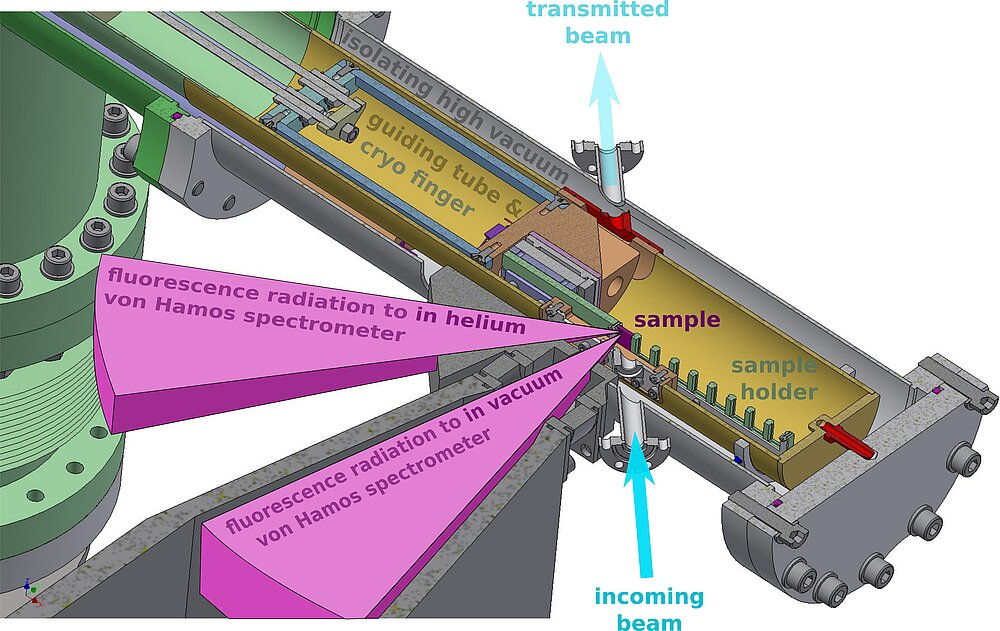
Resonant XES and XANES measurements are available with optional use of a double-crystal monochromator (DCM). The experimental setup shown in Fig. 3 is equipped with two in-house designed wavelength-dispersive von Hamos crystal spectrometers for analysis of fluorescent x-ray radiation.
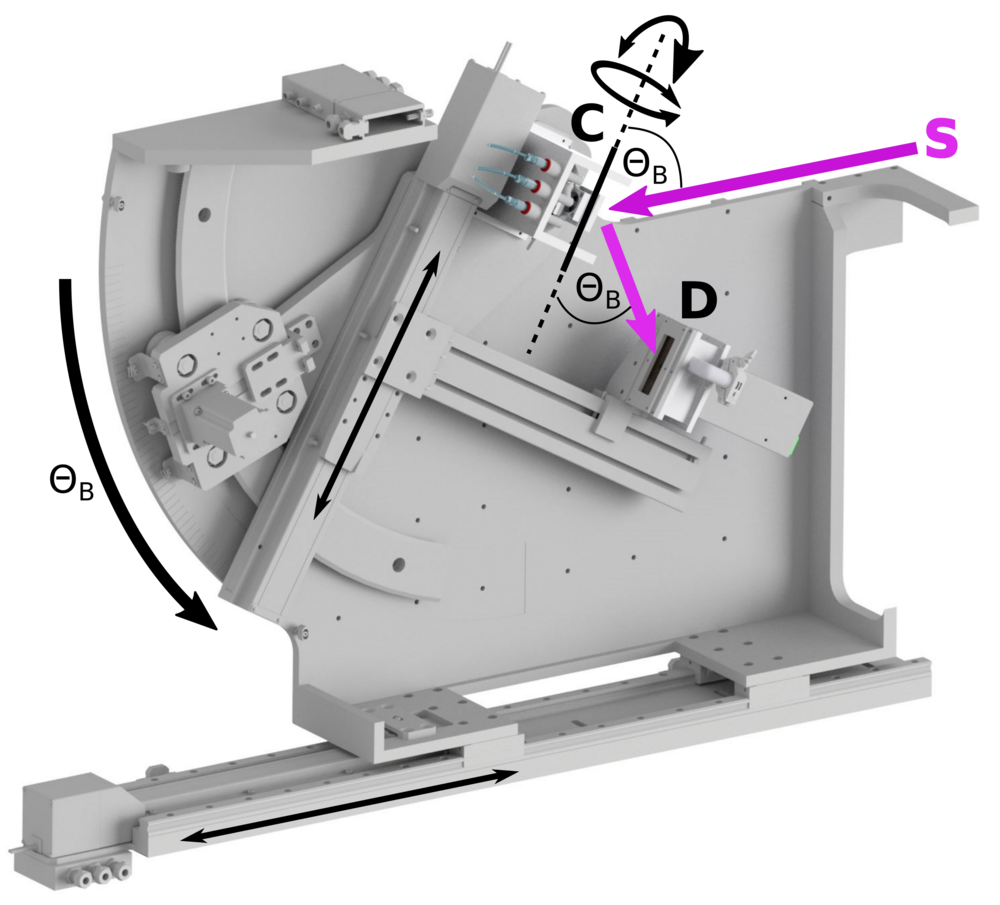
The first spectrometer is built to run at atmospheric pressure and covers 5-10 keV energy range (see Fig. 4). Using of a helium flight path the absorption of fluorescent photons can be reduced. The second spectrometer is placed inside a vacuum chamber and allows XES measurements at lower energies down to 2 keV. Many heterogeneous inorganic catalysts, as well as biological nitrogen reduction and water oxidation reactions, involve bimetallic clusters. Both spectrometers can be run independently, thus we will be able to record non-resonant XES spectra for two elements simultaneously, so called “two-color” experiments [6].
Taking advantage of a wavelength-dispersive analyzer to record the entire spectrum simultaneously and using fast x-ray position sensitive Mythen (1kHz) and Jungfrau (500Hz) detectors, we will be able to provide very attractive time resolved (0.1 -10 s) XES measurements [7].
In collaboration with the HZB sample environment group, we designed a cryo-cooled sample chamber to maintain the samples at 10 K temperature within a He atmosphere, in order to reduce radiation damages and avoid decomposition (Fig. 5).
The PINK setup incorporates a 4 meters long high-vacuum section, 3 area detectors, a cryo-cooled sample environment chamber, more than 30 stepping motors and piezodrives, beam diagnostic elements, other electronics and vacuum equipment. Our group develops control and acquisition software for the experimental station on the base of EPICS control system [8] and Python programming language. We also will provide software for preliminary data analysis.
References
[1] U. Bergmann and P. Glatzel, "X-ray emision spectroscopy" (2009) Photosynth. Res., 255
[2] M. A. Beckwith, M. Roemelt, M.-N. Collomb, C. DuBoc, T.-C. Weng U. Bergmann, P. Glatzel, F. Nesse and S. DeBeer, "Manganese Kb x-ray emission spectroscopy as a probe of metal-ligand interactions" (2011) Inorg. Chem, 50, 8397
[3] C. J. Pollock, K. Grubel, P. H. Holland, S. DeBeer, "Experimentally quantifying small-molecule bond activation using valence-to-core x-ray emission spectroscopy" (2013) J. of the Am. Chem. Soc., 135, 32, 11803
[4] C. Pollock and S. DeBeer, “Insights into the Geometric and Electronic Structure of Transition Metal Centers from Valence-to-Core X-ray Emission Spectroscopy” (2015) Acc. Chem. Res., 48 (11), 2967
[5] N. Lee, T. Petrenko, U. Bergmann, et al “Probing Valence Orbital Composition with Iron K beta X-ray Emission Spectroscopy” (2010) J. Am. Chem. Soc., 132 (28), 9715
[6] S. Gul, J. W. Desmond Ng. R. Alonso-Mori, J. Kern, et al., “Simultaneous detection of electronic structure changes from two elements of a bifunctional catalyst using wavelength-dispersive X-ray emission spectroscopy and in situ electrochemistry” (2015) Phys. Chem. Chem. Phys, 17, 8901
[7] J. Szlachetko, M. Nachtegaal, E. de Boni, M. Willimann, O. Safonova, J. Sa, G. Smolentsev, M. Szlachetko, J. A. van Bokhoven, J.-Cl. Dousse, J. Hoszowska, Y. Kayser, P. Jagodzinski, A. Bergamaschi, B. Schmitt, C. David, and A. Lücke, "A von Hamos x-ray spectrometer based on a segmented-type diffraction crystal for single-shot x-ray emission spectroscopy and time-resolved resonant inelastic x-ray scattering studies" (2012) Rev. Sci. Inst., 83, 103105
[8] EPICS control system: www.aps.anl.gov/epics/