Dr. Holger Ruland - Catalytic Technology
- Dr. Holger Ruland
- Group leader
- Catalytic Technology
- Heterogeneous Reactions
- +49 (0)208 306 - 3701
- holger.ruland(at)cec.mpg.de
- Room: 657
Vita
Publications
Full publications list | ORCID
Selected MPI CEC publications
- Gomez-Capiro, O., Ristig, S., Folke, J., Ruland, H. (2023). Challenges in Laboratory Catalytic Testing for Ammonia Decomposition under Industrially Relevant Conditions. Energy Technology, (xx): 2300996, pp. 1-11. doi:10.1002/ente.202300996.
- Hegen, O., Salazar Gomez, J. I., Gruenwald, C., Rettke, A., Sojka, M., Klucken, C., Pickenbrock, J., Filipp, J., Schlögl, R., Ruland, H. (2022). Bridging the Analytical Gap Between Gas Treatment and Reactor Plants in Carbon2Chem (R). CHEMIE INGENIEUR TECHNIK, (94), 1405-1412. doi:10.1002/cite.202200015.
- Ristig, S., Poschmann, M., Folke, J., Gomez-Capiro, O., Chen, Z., Sanchez-Bastardo, N., Schlögl, R., Heumann, S., Ruland, H. (2022). Ammonia Decomposition in the Process Chain for a Renewable Hydrogen Supply. Chemie-Ingenieur-Technik, (94), 1413-1425. doi:10.1002/cite.202200003.
- Hegen, O., Salazar Gomez, J. I., Schlögl, R., Ruland, H. (2022). The potential of NO+ and O-2(+center dot) in switchable reagent ion proton transfer reaction time-of-flight mass spectrometry. Mass Spectrometry Reviews, e21770. doi:10.1002/mas.21770.
- Folke, J., Dembele, K., Girgsdies, F., Song, H., Eckert, R., Reitmeier, S., Reitzmann, A., Schlögl, R., Lunkenbein, T., Ruland, H. (2022). Promoter effect on the reduction behavior of wuestite-based catalysts for ammonia synthesis. Catalysis Today, 387, 12-22. doi:10.1016/j.cattod.2021.03.013.
- Sanchez-Bastardo, N., Schlögl, R., Ruland, H. (2021). Response to Comment on "Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy". Industrial and Engineering Chemistry Research, 60(48), 17795-17796. doi:10.1021/acs.iecr.1c04435.
- Salazar Gomez, J. I., Sojka, M., Klucken, C., Schlögl, R., Ruland, H. (2021). Determination of trace compounds and artifacts in nitrogen background measurements by proton transfer reaction time-of-flight mass spectrometry under dry and humid conditions. Journal of Mass Spectrometry, 56(8): e4777, pp. 1-22. doi:10.1002/jms.4777.
- Fan, H., Folke, J., Liu, Z., Girgsdies, F., Ruland, H., Imlau, R., Ruland,H., Heumann, S., Granwehr, J., Eichel,R.- A., Schlögl, R., Frei, E.,Xing, H. (2021) Ultrathin 2D Fe-Nanosheets Stabilized by 2D Mesoporous Silica: Synthesis and Application in Ammonia Synthesis. ACS Appl. Mater. Inerfaces, 13(25), 30187-30197. doi:10.1021/acsami.1c06771
- Salazar Gómez, J.I., Klucken, C., Sojka, M., von der Waydbrink, G., Schlögl, R., Ruland, H. (2020). The HüGaProp‐Container: Analytical Infrastructure for the Carbon2Chem® Challenge Chemie Ingenieur Technik 92(10), 1514-1524. https://doi.org/10.1002/cite.202000101
- Sánchez-Bastardo, N., Schlögl, R., Ruland, H. (2020). Methane Pyrolysis for CO2‐Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness Chemie Ingenieur Technik 92(10), 1596-1609. https://doi.org/10.1002/cite.202000029
- Salazar Gómez, J.I., Takhtehfouladi, E.S., Schlögl, R., Ruland, H. (2020). Design and Implementation of a Gas Generating System for Complex Gas Mixtures and Calibration Gases Chemie Ingenieur Technik 92(10), 1574-1585. https://doi.org/10.1002/cite.202000110
- Laudenschleger, D., Ruland, H., Muhler, M. (2020). Identifying the nature of the active sites in methanol synthesis over Cu/ZnO/Al2O3 catalysts Nature Communications 11, 3898. https://doi.org/10.1038/s41467-020-17631-5
- He, J., Laudenschleger, D., Schittkowski, J., Machoke, A., Song, H., Muhler, M., Schlögl, R., Ruland, H. (2020). Influence of Contaminants in Steel Mill Exhaust Gases on Cu/ZnO/Al2O3 Catalysts Applied in Methanol Synthesis Chemie Ingenieur Technik 92(10), 1525-1532. https://doi.org/10.1002/cite.202000045
- Folke, M., Song, H., Schittkowski, J., Schlögl, R., Ruland, H. (2020). Oxygen Poisoning in Laboratory Testing of Iron‐Based Ammonia Synthesis Catalysts and its Potential Sources Chemie Ingenieur Technik 92(10), 1567-1573. https://doi.org/10.1002/cite.202000100
- Ruland, H., Song, H., Laudenschleger, D., Stürmer, S., Schmidt, S., He, J., Kähler, K., Muhler, M., Schlögl, R. (2020). CO2 hydrogenation with Cu/ZnO/Al2O3: A benchmark study ChemCatChem 12(12), 3216-3222. https://doi.org/10.1002/cctc.202000195
- Salazar Gómez, J.I., Klucken, C., Sojka, M., Masliuk, L., Lunkenbein, T., Schlögl, R., Ruland, H. (2019). Elucidation of artefacts in proton-transfer-reaction time-of-flight mass spectrometers Journal of Mass Spectrometry. https://doi.org/10.1002/jms.4479
- Schittkowski, J., Ruland, H., Laudenschleger, D., Girod, K., Kähler, K., Kaluza, S., Muhler, M., Schlögl, R. (2018). Methanol Synthesis from Steel Mill Exhaust Gases: Challenges for the Industrial Cu/ZnO/Al2O3 Catalyst Chemie Ingenieur Technik 90(10), 1419-1429. https://doi.org/10.1002/cite.201800017
- Song, H., Watermann, C., Laudenschleger, D., Yang, F., Ruland, H., Muhler, M. (2018). The effect of the thermal pretreatment on the performance of ZnO/Cr2O3 catalysts applied in high-temperature methanol synthesis Molecular Catalysis 451, 76-86. https://doi.org/10.1016/j.mcat.2017.10.033
Group members
Catalytic technology group
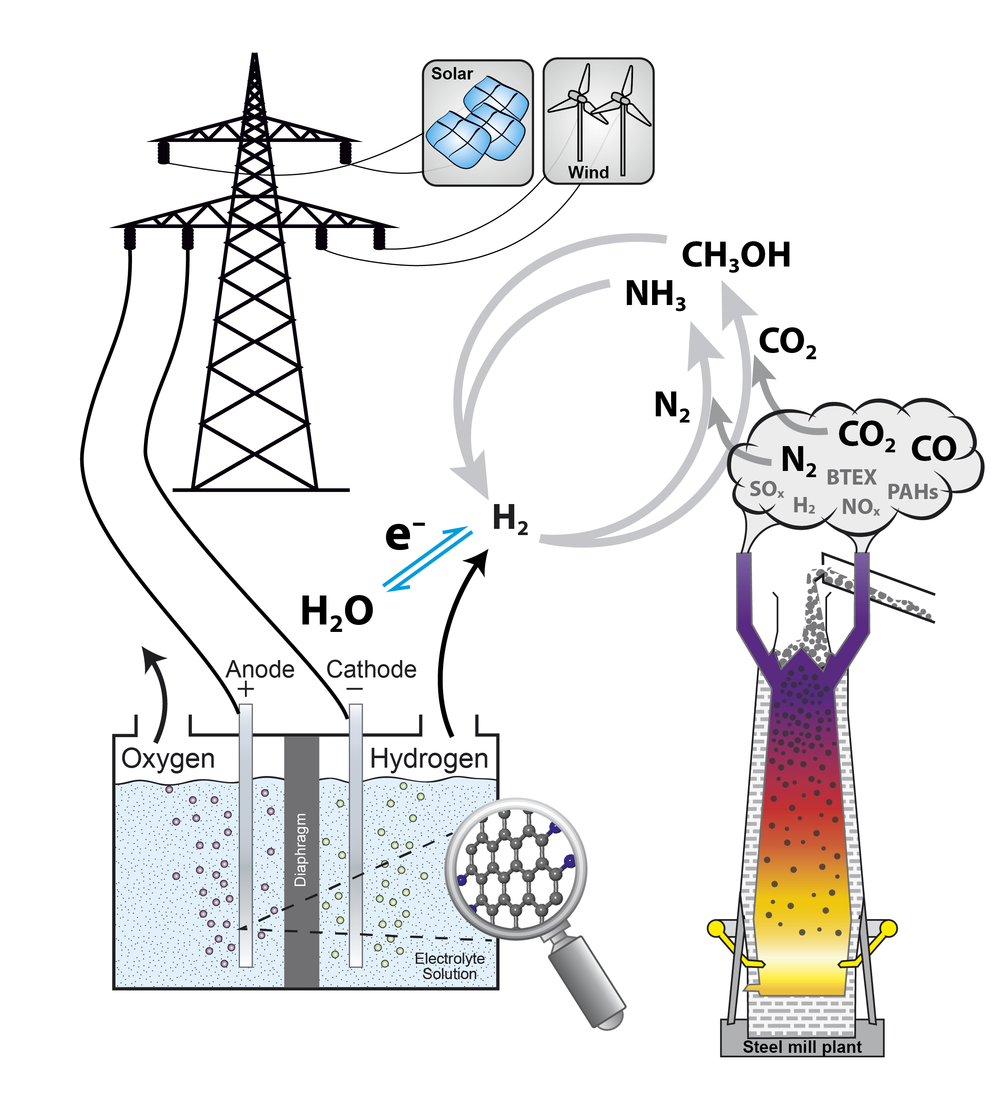
In the catalytictechnology group heterogeneously catalyzed reactions are investigated.Catalysis is of major importance for an energy-effective and sustainableconversion of reactants as it lowers the activation barrier of a chemicalreaction by changing its reaction mechanism. In heterogeneous catalysis,usually, the catalyst is a solid, whereas the reactants exist in thesurrounding fluid phase. Deeperunderstanding of catalytic processes can help to improve the efficiency ofreactions and, therefore, help to save resources and lower the overall energydemand.
In the catalytictechnology group reactions are focused, which are of interest in the scope ofenergy conversion and storage. Nowadays, solar, wind, and hydro energy areoften regarded as sustainable energy sources and can help to solve the shortageof hydro carbon-based energy sources in an environmentally friendly way.Electro-catalytic hydrogen production is investigated in the institute and isoften discussed as key technology to store the fluctuatingly appearingrenewable energies. Numerous techniques to store the hydrogen have beensuggested including the generation of ammonia and methanol, which areinvestigated in the catalytic technology group. Both substances offer theadvantage of high hydrogen contents and are easy to store as they arecondensable [1].
Analysis of exhaust gases of steel mill plants
Besides the electro-catalytic generated hydrogen different other chemical source materials can play an important role in chemical industry for the future. In the catalytic technology group the capability of the usage of industrial exhaust gases as potential hydrogen, carbon and/or nitrogen sources for methanol and ammonia synthesis is investigated. An extensive analysis of real exhaust gases from steel mill plants is performed for all kind of trace components including metals, sulfur-, nitrogen-, and chlorine-containing compounds, polycyclic aromatic hydrocarbons, as well as benzene, toluene, ethylbenzene, and xylene (BTEX-aromatics), and for many other gaseous components. Off-line exhaust gas analysis from blast furnace gas, coke oven gas, and converter gas were already performed. As the complex composition of the exhaust gases depends on the production conditions as well as the raw materials and is, therefore, time dependent, a detailed continuous analysis over longer time periods is mandatory. That is why in the catalytic technology group two mobile containers (a Lab-container and a Supply-container) were designed and constructed within the Project HügaProp (German: Hüttengasproperties), which was funded by the Federal Ministry of Education and Research (German: Bundesministerium für Bildung und Forschung BMBF, Förderkennzeichen 03EK3546). The Lab-container is a mobile high-end gas analysis laboratory housed in a container to analyze the steel mill gases directly on-site. It is equipped with a new generation PTR-SRI-QiTOF-MS for high performance analysis and reactor set-ups for catalytic testing in methanol. The exhaust gas analysis of the steel mill plants will be performed within the project Carbon2Chem in the subproject L0, which is also funded by the Federal Ministry of Education and Research (German: Bundesministerium für Bildung und Forschung BMBF, Förderkennzeichen 03EK3038C).
Methanol synthesis
Nowadays methanolis produced from feed gas mixtures containing CO, CO2, and H2in a conventional, continuously performed process using a Cu/ZnO/Al2O3catalyst. As the Cu/ZnO/Al2O3 system is also active inthe hydrogenation of pure CO2 without CO in the feed gas, themethanol synthesis starting from CO2/H2 mixtures is apromising candidate to help to stabilize the CO2 level of theatmosphere. Simultaneously, a re-incorporation of the green-house gas CO2into the value added chain of the industrial chemistry can be achieved asmethanol is the most important alcohol with respect to its production volumeand numerous applications. A future sustainable and environmental friendlyproduction route from exhaust gases renders a deeper understanding of the CO2hydrogenation and an expansion of the known parameter space of a Cu-basedcatalyst important. The usage of CO2-rich feed gases, possiblywithout any CO at all, and dynamic operation conditions pose typical challengesfor such a process and are investigated in the catalytic technology group. Theinfluence of parameters such as pressure, temperature and space velocity isinvestigated with respect to the reaction performance to help to establish afundament for the usage of exhaust gases of e.g. steel mill plants [2].
In the catalytictechnology group reactivity studies of the CO2 hydrogenation areperformed within the project Carbon2Chemin the subproject L2 (ProMeOH -Methanolsynthese aus Hüttengasen), which is funded by the Federal Ministryof Education and Research (BMBF, Förderkennzeichen 03EK3039D). The reactorapplied houses sufficient catalyst to determine the productivity by volumetricanalysis of condensate and high-precision GC-MS product analysis. Furthermore,the influence of impurities of the exhaust gases on the catalyst activity andstability is investigated. To find out to which extent gas purification isnecessary, systematic reactivity and stability studies in methanol synthesishave been started. Feed gases consist of COx, hydrogen, andadditionally trace components, which are usually found in the exhaust gases,like benzene as a representative of BTEX-aromatics.
Ammonia synthesis
Ammonia synthesisrepresents an additional way to store hydrogen produced with renewable energysources. It is well known that ammonia synthesis is a structure sensitivereaction [3]. Nevertheless, there is still a large pressure and material gapbetween activity data at industrial relevant conditions and surface scienceapproaches. That is why it was planned to investigate a systematic series ofcatalysts with different surface structures and to correlate their activity totheir structure. For this purpose the catalytic technology group investigatesthe activity and stability of alternative Fe-based catalyst systems in akinetic flow set-up [4].Another key point in the alternativecatalyst preparation routes is the circumvention of the high-temperature meltingprocess of the iron oxide precursor. Such a “green” synthesis allows studyingthe interplay of morphology and promoter chemistry in the formation of theactive iron species.
A special reactorwas constructed allowing the disassembly of the catalyst inside a glove boxwithout contact to the ambient for ex-situ characterization methods. This mayhelp to elucidate whether surface, subsurface, or bulk nitride species aregenerated during the reaction, as the active phase of the Fe-based catalystunder industrially used conditions is still unclear [5]. The very lowconversion in ammonia synthesis at mbar pressure precludes direct in-situanalysis and the extreme air-sensitivity of the activated catalysts representsa major hurdle in getting insight into the chemistry of the active form ofammonia iron.
References
[1] Jensen, J. O.; Vestbø, A. P.; Li, Q.; Bjerrum, N. J., J. Alloys Compd. (2007) 446-447, 723.
[2] Bukhtiyarova, M., Lunkenbein, T., Kähler, K., Schlögl, R., Catal. Lett. (2017) DOI: 10.1007/s10562-016-1960-x.
[3] Schlögl, R., Ammonia Synthesis. In Handbook of Heterogeneous Catalysis, 2 ed.; Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J., Eds. Weinheim (2008).
[4] Ortega, K.F., Rein, D., Lüttmann,, C., Heese, J., Özcan, F., Heidelmann, M., Folke, J., Kähler, K., Schlögl, R., Behrens, M., ChemCatChem, DOI: 10.1002/cctc.201601355.
[5] Kandemir, T., Schuster, M.E., Senyshyn, A., Behrens, M., Schlögl, R., Angew. Chem. Int. Ed. (2013) 52, 12723.
[6] Delgado, J., Chen, X., Frank, B., Su, D.S. , Schlögl, R., Catal. Today (2012) 186, 93.
[7] Rinaldi, A., Zhang, J., Frank, B., Su, D.S., Hamid, S.B.A., Schlögl, R. ChemSusChem (2010) 3, 254.




