Dr. Alexis Bordet - Multifunctional Catalytic Systems
- Dr. Alexis Bordet
- Group leader
- Multifunctional Catalytic Systems
- Molecular Catalysis
- +49 (0)208 306 - 3167
- alexis.bordet(at)cec.mpg.de
- Room: 471
Vita
| Engineer | University of Toulouse/INP ENSIACET (2010-2013) |
| M. Sc. | University of Toulouse (2012-2013) |
| Ph.D. | University of Toulouse/LPCNO (Dr. Bruno Chaudret), France (2013-2016) |
| Post-Doc | RWTH Aachen University (Prof. Dr. Walter Leitner), Germany (2017-2018) |
| Group Leader | 'Multifunctional Catalytic Systems', MPI CEC (seit 2018) |
Publications
Full publications list | ORCID
Selected MPI CEC publications
- Sodreau, A., Zahedi, H. G., Dervişoğlu, R., Kang, L., Menten, J., Zenner, J., Terefenko, N., DeBeer, S., Wiegand, T., Bordet, A., Leitner, W. A Simple and Versatile Approach for the Low-Temperature Synthesis of Transition Metal Phosphide Nanoparticles from Metal Chloride Complexes and P(SiMe3)3. Adv. Mater. 2023, 2306621. https://doi.org/10.1002/adma.202306621
- Levin, N., Goclik, L., Walshus, H., Antil, N., Bordet, A., Leitner, W. Decarboxylation and Tandem Reduction/Decarboxylation Pathways to Substituted Phenols from Aromatic Carboxylic Acids using Bimetallic Nanoparticles on Supported Ionic Liquid Phases as Multifunctional Catalysts. J. Am. Chem. Soc. 2023, 145, 22845-22854. https://doi.org/10.1021/jacs.3c09290
- Zhang, Y., El Sayed, S., Kang, L., Sanger, M., Wiegand, T., Jessop, P. G., DeBeer, S., Bordet, A., Leitner, W. Adaptive Catalysts for the Selective Hydrogenation of Bicyclic Heteroaromatics using Ruthenium Nanoparticles on a CO2-Responsive Support. Angew. Chem. Int. Ed. 2023,e202311427. https://doi.org/10.1002/anie.202311427
- Bordet, A., Leitner, W. Adaptive Catalytic Systems for Chemical Energy Conversion. Angew. Chem. Int. Ed. 2023, e202301956. https://doi.org/10.1002/anie.202301956
- Han, C., Zenner, J., Johny, J., Kaeffer, N., Bordet, A., Leitner, W. Electrocatalytic Hydrogenation of Alkenes with Pd/Carbon Nanotubes at an Oil-Water Interface. Nat. Catal. 2022, 5, 1110-1119. https://doi.org/10.1038/s41929-022-00882-4
- Kalsi, D., Louis Anandaraj, J. L., Durai, M., Weidenthaler, C., Emondts, M., Nolan, S. P., Bordet, A., Leitner, W. One-Pot Multicomponent Synthesis of Allyl and Alkylamines Using a Catalytic System Composed of Ruthenium Nanoparticles on Copper N-Heterocyclic Carbene-Modified Silica. ACS Catal. 2022, 12, 14902-14910. https://doi.org/10.1021/acscatal.2c04044
- Lin, S.-H., Hetaba, W., Chaudret, B., Leitner, W., Bordet, A. Copper-Decorated Iron Carbide Nanoparticles Heated by Magnetic Induction as Adaptive Multifunctional Catalysts for the Selective Hydrodeoxygenation of Aldehydes. Adv. Energy Mater. 2022, 2201783.https://doi.org/10.1002/aenm.202201783
- Kreissl, H., Jin, J., Lin, S.-H., Schüette, D., Störtte, S., Levin, N., Chaudret, B., Vorholt, A. J., Bordet, A., Leitner, W. Commercial Cu2Cr2O5 Decorated with Iron Carbide Nanoparticles as Multifunctional Catalyst for Magnetically Induced Continuous Flow Hydrogenation of Aromatic Ketones. Angew. Chem. Int. Ed. 2021, 60, 26639-26646. https://doi.org/10.1002/anie.202107916
- Bordet, A., Leitner, W. Metal Nanoparticles Immobilized on Molecularly Modified Surfaces: Versatile Catalytic Systems for Controlled Hydrogenation and Hydrogenolysis. Acc. Chem. Res. 2021, 54, 2144-2157 https://doi.org/10.1021/acs.accounts.1c00013
Group members
Research in the Team “Multifunctional Catalytic Systems”
In the “Multifunctional Catalytic Systems” team, we work on the development of catalytic systems capable of activating and transferring molecular hydrogen (H2), and that we use for various transformations, including selective hydrogenation, hydrodeoxygenation, hydrogenolysis, and decarboxylation reactions.
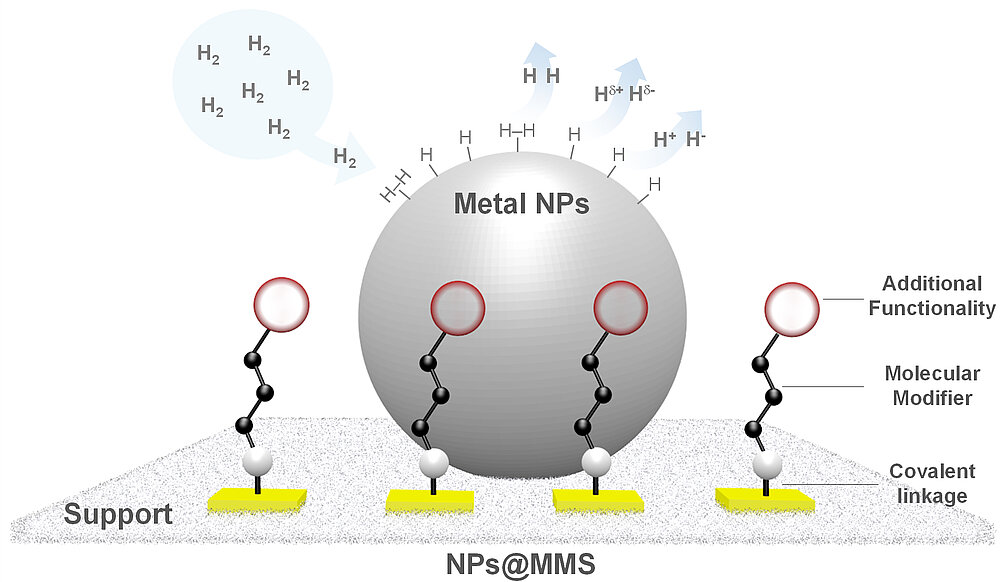
Metal Nanoparticles Immobilized on Molecularly Modified Surfaces (NPs@MMS)
One of our research lines consists in the synthesis, characterization, and application in catalysis of metallic nanoparticles immobilized on molecularly modified surfaces (NPs@MMS, Figure 1). We are especially interested in combining molecular design (molecular modifier structure), nanoparticle design, and choice of support material to produce innovative catalytic systems providing control over the activation mode of H2 (e.g. homolytic, polarized, heterolytic).
Molecular modifiers commonly used in our group include small organic molecules, ionic liquids, and polymers. Metal nanoparticles (e.g. Mn, Fe, Co, Ni, Ru, Rh, Pt and bimetallic) are synthesized directly in the MMS from organometallic precursors under mild conditions. This organometallic approach provides a fine control over the nanoparticles size, dispersion, and in the case of bimetallic nanoparticles, composition. In addition, this insures a close contact between the metal NPs and the molecular modifiers, leading to high NPs stability and strong synergistic effects.
Relevant recent publications:
- Accounts: Bordet, A., Leitner, W. Metal Nanoparticles Immobilized on Molecularly Modified Surfaces: Versatile Catalytic Systems for Controlled Hydrogenation and Hydrogenolysis. Acc. Chem. Res. 2021, 54, 2144-2157 https://doi.org/10.1021/acs.accounts.1c00013
- D. Kalsi et al. One-Pot Multicomponent Synthesis of Allyl and Alkylamines Using a Catalytic System Composed of Ruthenium Nanoparticles on Copper N-Heterocyclic Carbene-Modified Silica. ACS Catal. 2022, 12, 14902-14910. https://doi.org/10.1021/acscatal.2c04044
- S. J. Louis Anandaraj et al. Catalytic Hydrogenation of CO2 to Formate Using Ruthenium Nanoparticles Immobilized on Supported Ionic Liquid Phases. Small 2023, 2206806. https://doi.org/10.1002/smll.202206806
- N. Levin et al. Decarboxylation and Tandem Reduction/Decarboxylation Pathways to Substituted Phenols from Aromatic Carboxylic Acids using Bimetallic Nanoparticles on Supported Ionic Liquid Phases as Multifunctional Catalysts. J. Am. Chem. Soc. 2023, 145, 22845-22854. https://doi.org/10.1021/jacs.3c09290
Adaptive Catalytic Systems
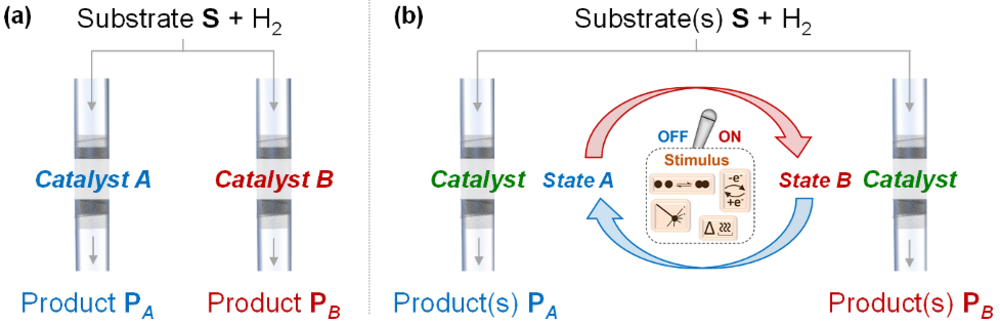
While the developed NPs@MMS catalysts present outstanding properties regarding their dedicated tasks, their performance is typically optimized to remain static (Figure 2a). However, flexibility and adaptivity will become increasing important in the future to cope with the dynamics of alternative energy resources, quality variations of chemical feedstocks, and to enable customized and decentralized production.
In this context, we work on the design of adaptive catalytic systems (Figure 2b), that we recently defined as “capable of adjusting or being adjusted into different states of their performance in response to dynamic changes in the reactive environment”, and that provide opportunities to adjust product selectivity (i.e. adaptivity in product formation) and/or catalytic activity (i.e. adaptivity to intermittent electricity supply), in a reversible, rapid, and robust manner (R3 rule).
Scientific perspective on the topic:
- A. Bordet and W. Leitner, Adaptive Catalytic Systems for Chemical Energy Conversion. Angew. Chem. Int. Ed. 2023, e202301956. https://doi.org/10.1002/anie.202301956
Adaptivity in product formation
Our work on the development of catalytic systems with adaptive selectivity relies on the NPs@MMS catalyst design, in which we introduce specific functionalities in our molecular modifiers that take part in chemical reactions used to modify metal active sites and impact on intermediates and transition states of the catalytic cycle, thereby potentially opening or closing certain pathways. Recent advances in this field involve for example the use of CO2 as a molecular trigger, in combination with amine-functionalized multifunctional catalytic systems.
Relevant recent publications:
- A. Bordet et al. Selectivity Control in Hydrogenation through Adaptive Catalysis using Ruthenium Nanoparticles on a CO2-Responsive Support. Nat. Chem. 2021, 13, 916-922. https://doi.org/10.1038/s41557-021-00735-w
- Y. Zhang et al. Adaptive Catalysts for the Selective Hydrogenation of Bicyclic Heteroaromatics using Ruthenium Nanoparticles on a CO2-Responsive Support. Angew. Chem. Int. Ed. 2023, e202311427. https://doi.org/10.1002/anie.202311427
Adaptivity to intermittent electricity supply
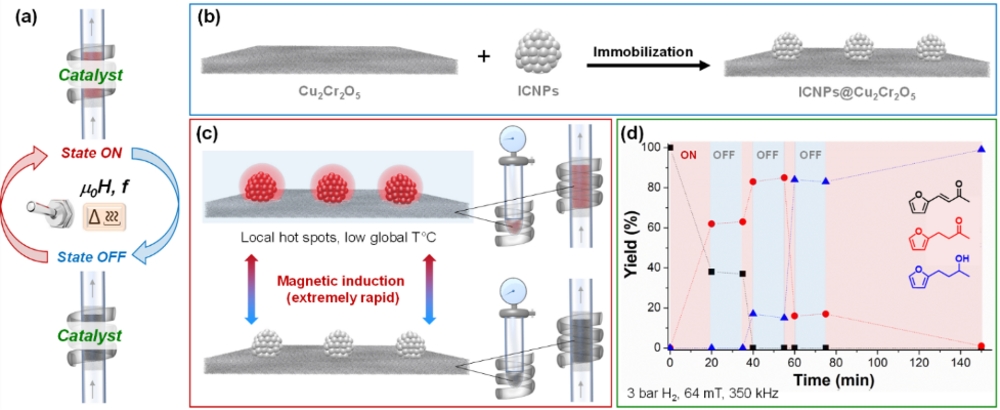
Adaptivity to intermittent electricity supply implies the possibility to turn catalytic activity ON and OFF in a very rapid and reversible manner. While conventional heating is a relatively slow process, magnetic induction heating provides an instantaneous and highly controlled way to provide thermal energy input at well-defined confinements. In our group, we are developing multifunctional catalysts integrating active materials with magnetic heating capabilities in order to beneficiate from an extremely localized, rapid, and energy efficient catalyst heating (Figure 2). For that, we work with magnetic nanoparticles possessing excellent heating power under magnetic induction (specific absorption rate SAR), and in particular iron carbide nanoparticles (ICNPs) that we use either as heating agents for heterogeneous catalysts (see example in Figure 2), or directly as magnetically-responsive support materials for catalytically active metal nanoparticles.
Relevant recent publications:
- H. Kreissl, S.-H. Lin et al. Commercial Cu2Cr2O5 Decorated with Iron Carbide Nanoparticles as Multifunctional Catalyst for Magnetically Induced Continuous Flow Hydrogenation of Aromatic Ketones. Angew. Chem. Int. Ed. 2021, 60, 26639-26646. https://doi.org/10.1002/anie.202107916
- S.-H. Lin et al. Copper-Decorated Iron Carbide Nanoparticles Heated by Magnetic Induction as Adaptive Multifunctional Catalysts for the Selective Hydrodeoxygenation of Aldehydes. Adv. Energy Mater. 2022, 2201783. https://doi.org/10.1002/aenm.202201783
Other Topics of Interest
Electrocatalytic hydrogenation using NPs@MMS and Pickering Emulsions
Relevant recent publication:
- C. Han et al. Electrocatalytic Hydrogenation of Alkenes with Pd/Carbon Nanotubes at an Oil-Water Interface. Nat. Catal. 2022, 5, 1110-1119. https://doi.org/10.1038/s41929-022-00882-4
Development of Metal Phosphide Nanoparticles
- A. Sodreau, H. G. Zahedi et al. A Simple and Versatile Approach for the Low-Temperature Synthesis of Transition Metal Phosphide Nanoparticles from Metal Chloride Complexes and P(SiMe3)3. Adv. Mater. 2023, 2306621. https://doi.org/10.1002/adma.202306621