Dr. Alexander Schnegg - EPR Research Group
- Dr. Alexander Schnegg
- Research Group Leader
- EPR Research Group
- +49 (0)208 306 - 3526
- alexander.schnegg(at)cec.mpg.de
- Room: 211
Vita
| Dipl.-Phys. | Free University Berlin (1998) |
| Dr. rer. nat. | Institut für Experimentalphysik, Free University Berlin (1999-2003) |
| Postdoc | Max Planck Institute for Bioinorganic Chemistry; today: MPI CEC (2004-2005) |
| Postdoc | Helmholtz Zentrum Berlin für Materialien und Energie (HZB) (2006-2013) |
| Staff Scientist | HZB's EPR lab (2013-2018) |
| Adjunct Professor | Monash University, Melbourne, Australia (since 2016) |
| Research group leader | MPI CEC (since 2018) |
Publications
Selected MPI CEC publications
- Mato, M., Bruzzese, P. C., Takahashi, F., Leutzsch, M., Reijerse, E. J., Schnegg, A., Cornella, J. (2023). Oxidative Addition of Aryl Electrophiles into a Red-Light-Active Bismuthinidene. Journal of the American Chemical Society, 145(34), 18742-18747. doi:10.1021/jacs.3c06651.
- Tran, V. A., Teucher, M., Galazzo, L., Sharma, B., Pongratz, T., Kast, S. M. M., Marx, D., Bordignon, E., Schnegg, A., Neese, F. (2023). Dissecting the Molecular Origin of g-Tensor Heterogeneity and Strain in Nitroxide Radicals in Water: Electron Paramagnetic Resonance Experiment versus Theory. The Journal of Physical Chemistry A, 127(31), 6447-6466. doi:10.1021/acs.jpca.3c02879.
- Tretiakov, S., Lutz, M., Titus, C. J., de Groot, F., Nehrkorn, J., Lohmiller, T., Holldack, K., Schnegg, A., Tarrago, M. F. X., Zhang, P., Ye, S., Aleshin, D., Pavlov, A., Novikov, V., Moret, M.-E. (2023). Homoleptic Fe(III) and Fe(IV) Complexes of a Dianionic C-3-Symmetric Scorpionate. Inorganic Chemistry, (62), 101613-10625. doi:10.1021/acs.inorgchem.3c00871.
- Rams, M., Lohmiller, T., Böhme, M., Jochim, A., Foltyn, M., Schnegg, A., Plass, W., Näther, C. (2023). Weakening the Interchain Interactions in One Dimensional Cobalt(II) Coordination Polymers by Preventing Intermolecular Hydrogen Bonding. INORGANIC CHEMISTRY, 62(26), 10420-10430. doi:10.1021/acs.inorgchem.3c01324.
- Lau, K., Niemann, F., Abdiaziz, K., Heidelmann, M., Yang, Y., Tong, Y., Fechtelkord, M., Schmidt, T. C., Schnegg, A., Campen, R. K., Peng, B., Muhler, M., Reichenberger, S., Barciowski, S. (2023). Differentiating between Acidic and Basic Surface Hydroxyls on Metal Oxides by Fluoride Substitution: A Case Study on Blue TiO2 from Laser Defect Engineering. Angewandte Chemie, International Edition in English, (62): e20221396, pp. 1-12. doi:10.1002/anie.202213968.
- Yang, X., Reijerse, E. J., Nöthling, N., SantaLucia, D., Leutzsch, M., Schnegg, A., Cornellà, J. (2023). Synthesis, Isolation, and Characterization of Two Cationic Organobismuth(II) Pincer Complexes Relevant in Radical Redox Chemistry. Journal of the American Chemical Society, (145), 5618_5623. doi:10.1021/jacs.2c12564.
- Yang, X., Reijerse, E.J., Nöthling, N., SantaLucia, D.J., Leutzsch, M., Schnegg, A., Cornella, J., (2023). Synthesis, Isolation, and Characterization of Two Cationic Organobismuth(II) Pincer Complexes Relevant in Radical Redox Chemistry. (2023) Journal of the American Chemical Society. https://doi.org/10.1021/jacs.2c12564.
- Lau, K., Niemann, F., Abdiaziz, K., Heidelmann, M., Yang, Y., Tong, Y., Fechtelkord, M., Schmidt, T. C., Schnegg, A., Campen, R. K., Peng, B., Muhler, M., Reichenberger, S., Barcikowski, S. (2023). Differentiating between Acidic and Basic Surface Hydroxyls on Metal Oxides by Fluoride Substitution: A Case Study on Blue TiO2 from Laser Defect Engineering. Angewandte Chemie International Edition, e202213968. https://doi.org/10.1002/anie.202213968.
- Larsen, E. M. H., Bonde, N. A., Weihe, H., Ollivier, J., Vosch, T., Lohmiller, T., Holldack, K., Schnegg, A., Perfetti, M., Bendix, J. (2023). Experimental assignment of long-range magnetic communication through Pd & Pt metallophilic contacts. Chemical Science, 14 (2), 266-276. doi:10.1039/d2sc05201f.
- Pohle, M.H., Böhme, M., Lohmiller, T., Ziegenbalg, S., Blechschmidt, L.; Görls, H., Schnegg, A., Plass, W. (2023). Magnetic Anisotropy and Relaxation of Pseudotetrahedral [N2O2] Bis-Chelate Cobalt(II) Single-Ion Magnets Controlled by Dihedral Twist Through Solvomorphism. Chemistry a European Journal,(29)1-7 e202202966. https://doi.org/10.1002/chem.202202966
- Sharma, M. K., Chabbra, S., Wölper, C., Weinert, H. M., Reijerse, E. J., Schnegg, A., Schulz, S. (2022). Modulating the frontier orbitals of L(X)Ga-substituted diphosphenes [L(X)GaP](2) (X = Cl, Br) and their facile oxidation to radical cations. Chemical Science, 13(43), 12643-12650. doi:10.1039/d2sc04207j.
- Yang, X., Reijerse, E. J., Bhattacharyya, K., Leutzsch, M., Kochius, M., Nöthling, N., Busch, J., Schnegg, A., Auer, A. A., Cornella, J. (2022). Radical Activation of N-H and O-H Bonds at Bismuth(II). Journal of the American Chemical Society, (144), 16535-16544. doi:10.1021/jacs.2c05882.
- Lohmiller, T., Spyra, C.-J., Dechert, S., Demeshko, S., Bill, E., Schnegg, A., Meyer, F. (2022). Antisymmetric Spin Exchange in a mu-1,2-Peroxodicopper(II) Complex with an Orthogonal Cu-O-O-Cu Arrangement and S = 1 Spin Ground State Characterized by THz-EPR. JACS Au, 2(5), 1134-1143. doi:10.1021/jacsau.2c00139.
- Teucher, M., Sidabras, J. W., Schnegg, A. (2022). Milliwatt three- and four-pulse double electron electron resonance for protein structure determination. Physical Chemistry Chemical Physics, (24), 12528-12540. doi:10.1039/d1cp05508a.
- Chatterjee, S., Harden, I., Bistoni, G., Castillo, R. G., Chabbra, S., van Gastel, M., Schnegg, A., Bill, E., Birrell, J. A., Morandi, B., Neese, F., DeBeer, S. (2022). A Combined Spectroscopic and Computational Study on the Mechanism of Iron-Catalyzed Aminofunctionalization of Olefins Using Hydroxylamine Derived N-O Reagent as the "Amino" Source and "Oxidant". Journal of the American Chemical Society, 144(6), 2637-2656. doi:10.1021/jacs.1c11083.
- Bonke, S.A., Risse, T., Schnegg A., Brückner A., (2021) In situ electron paramagnetic resonance spectroscopy for catalysis; Nature Review Methods Primers 2021 1 (1), 33. https://doi.org/10.1038/s43586-021-00031-4
- Liu, C., Geer, A. M., Webber, C., Musgrave, C., Gu, S., Johnson, G., Dickie, D. A., Chabbra, S., Schnegg, A., Zhou, H., Sun, C.-J., Hwang, S., Goddard III, W. A., Zhang, S., Gunnoe, T. B. (2021). Immobilization of "Capping Arene" Cobalt(II) Complexes on Ordered Mesoporous Carbon for Electrocatalytic Water Oxidation. ACS Catalysis, (11), 15068-1508 DOI: 10.1021/acscatal.1c04617
- Shin, D., Bhandari, S., Tesch, M. F., Bonke, S. A., Jaouen, F., Chabbra, S., Pratsch, C., Schnegg, A., Mechler, A. K. (2022). Reduced formation of peroxide and radical species stabilises iron-based hybrid catalysts in polymer electrolyte membrane fuel cells. Journal of Energy Chemistry, 65, 433-438. doi:10.1016/j.jechem.2021.05.047 .
- Büker, J., Alkan, B., Chabbra, S., Kochetov, N., Falk, T., Schnegg, A., Schulz,C., Wiggers, H., Muhler, M., Peng, B. (2021). Liquid-Phase Cyclohexene Oxidation with O-2 over Spray-Flame-Synthesized La1-xSrxCoO3 Perovskite Nanoparticles. SI, 27(68), 16912-16923. doi:10.1002/chem.202103381.
- Nehrkorn, J. P., Valuev, I. A., Kiskin, M. A., Bogomyakov, A. S., Suturina, E. A., Sheveleva, A. M., Ovcharenko, V., Holldack, K.,Herrmann, C., Fedin, M. V., Schnegg, A., Veber, S.L. (2021) Easy-plane to easy-axis anisotropy switching in a Co(ii) single-ion magnet triggered by the diamagnetic lattice. Journal of Materials Chemistry C, 9(30), 9446, 1-7. doi:10.1039/d1tc01105g.
- Ott, J. C., Suturina, E. A., Kuprov, I., Nehrkorn, J., Schnegg, A., Enders, M., Lutz, H.G. (2021). Observability of paramagnetic NMR signals at over 10 000 ppm chemical shifts. Angewandte Chemie, International Edition, (60), 22856-22864. doi:10.1002/anie.202107944.
- Zhao, G., Bonke, S. A., Schmidt, S., Wang, Z., Hu, B., Falk, T., Hu, Y., Rath, T ., Xia, W ., Peng, B., Schnegg, A., Weng, Y., Muhler, M.(2021). Highly Efficient and Selective Aerobic Oxidation of Cinnamyl Alcohol under Visible Light over Pt-Loaded NaNbO3 Enriched with Oxygen Vacancies by Ni Doping. ACS Sustainable Chemistry & Engineering, 9(15), 5422-5429. doi:10.1021/acssuschemeng.1c00460.
- Shin, D., Bhandari, S., Tesch, M. F., Bonke, S. A., Jaouen, F., Chabbra, S., Pratsch, C., Schnegg, A., Mechler, A.K. (2022). Reduced formation of peroxide and radical species stabilises iron-based hybrid catalysts in polymer electrolyte membrane fuel cells. Journal of Energy Chemistry, 65, 433-438. doi.org/10.1016/j.jechem.2021.05.047 .
- Bone, A. N., Widener, C. N., Moseley, D. H., Liu, Z., Lu, Z., Cheng, Y., Daemen, L. L, Ozerov, M.,Telser, J., Thirunavukkuarasu, K., Smirnov, D., Greer, S. M., Hill, Stephen., Krzystek, J., Holldack, K., Aliabadi, A., Schnegg, A., Dunbar, K. R., Xue, Zi-Ling (2021). Applying Unconventional Spectroscopies to the Single-Molecule Magnets, Co(PPh3)(2)X-2 (X=Cl, Br, I): Unveiling Magnetic Transitions and Spin-Phonon Coupling. Chemistry – A European Journal, 27, 11110. doi:10.1002/chem.202100705.
- Tarrago, M., Römelt, C., Nehrkorn, J., Schnegg, A., Neese, F., Bill, E., Ye, S. (2021). Experimental and Theoretical Evidence for an Unusual Almost Triply Degenerate Electronic Ground State of Ferrous Tetraphenylporphyrin. Inorganic chemistry, 60(7), 4966-4985. doi:10.1021/acs.inorgchem.1c00031.
- Nehrkorn, J. P., Greer, S. M., Malbrecht, B. J., Anderton, K. J., Azat Aliabadi, K., Schnegg, A., Holldack, K., Herrmann, C., Betley, T., Stoll, S., Hill, S. (2021). Spectroscopic Investigation of a Metal–Metal-Bonded Fe6 Single-Molecule Magnet with an Isolated S = 19/2 Giant-Spin Ground State. Inorganic Chemistry, 60(7), 4610-4622. doi:10.1021/acs.inorgchem.0c03595.
- Pavlov, A.A., Nehrkorn, J., Zubkevich, S.V., Fedin, M.V., Holldack, K., Schnegg, A., Novikov, V.V. (2020). A Synergy and Struggle of EPR, Magnetometry and NMR: A Case Study of Magnetic Interaction Parameters in a Six-Coordinate Cobalt(II) Complex Inorganic Chemistry 59(15), 10746-10755. https://doi.org/10.1021/acs.inorgchem.0c01191.
- Viciano-Chumillas, M., Blondin, G., Clémanceym N., Krzystek, J., Ozerov, M., Armentano, D., Schnegg, A., Lohmiller, T., Telser, J., Lloret, F., Cano, J. (2020). Single‐Ion Magnetic Behaviour in an Iron(III) Porphyrin Complex: A Dichotomy Between High‐Spin and 5/2−3/2 Spin Admixture Chemistry – A European Journal 26(62), 14242-14251. https://doi.org/10.1002/chem.202003052.
- Jochim, A., Lohmiller, T., Rams, M., Böhme, M., Ceglarska, M., Schnegg, A., Plass, W., Näther, C. (2020). Influence of the Coligand onto the Magnetic Anisotropy and the Magnetic Behavior of One-Dimensional Coordination Polymers Inorganic Chemistry 59(13), 8971-8982. https://doi.org/10.1021/acs.inorgchem.0c00815.
- Lin, Y.-H., Kutin, Y., van Gastel, M., Bill, E., Schnegg, A., Ye, S., Lee, W.-Z. (2020). A Manganese(IV)-Hydroperoxo Intermediate Generated by Protonation of the Corresponding Manganese(III)-Superoxo Complex Journal of the American Chemical Society 11(24), 15068-15082. https://doi.org/10.1021/jacs.0c02756.
- Böhme, M., Jochim, A., Rams, M., Lohmiller, T., Suckert, S., Schnegg, A., Plass, W., Näther, C. (2020). Variation of the Chain Geometry in Isomeric 1D Co(NCS)2 Coordination Polymers and Their Influence on the Magnetic Properties Inorganic Chemistry 59(8), 5325-5338. https://doi.org/10.1021/acs.inorgchem.9b03357.
- Ma, Y., Pang, Y., Chabbra, S., Reijerse, E.J., Schnegg, A., Niski, J., Leutzsch, M., Cornella, J. (2020). Radical C‒N Borylation of Aromatic Amines Enabled by a Pyrylium Reagent Chemistry – A European Journal 26(17), 3734-3743. https://doi.org/10.1002/chem.202000412.
- Krzystek, J., Schnegg, A., Aliabadi, A., Holldack, K., Stoian, S.A., Ozarowski, A., Hicks, S.D., Abu Omar, M.M., Thomas, K.E., Ghosh, A., Caulfield, K.P., Tonzetich, Z.J., Telser, J. (2020). Advanced Paramagnetic Resonance Studies on Manganese and Iron Corroles with a Formal d4 Electron Count Inorganic Chemistry 59(2), 1075-1090. https://doi.org/10.1021/acs.inorgchem.9b02635.
- Li, J., Chen, J., Sang, R., Ham, W.-S., Plutschack, M.B., Berger, F., Chabbra, S., Schnegg, A., Genicot, C., Ritter, T. (2020). Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination Nature Chemistry 12, 56-62. https://doi.org/10.1038/s41557-019-0353-3.
- Rams, M., Jochim, A., Böhme, M., Lohmiller, T., Ceglarska, M., Rams, M.M., Schnegg, A., Plass, W., Näther, C. (2020). Single‐Chain Magnet Based on Cobalt(II) Thiocyanate as XXZ Spin Chain Chemistry – A European Journal 26(13), 2837-2851. https://doi.org/10.1002/chem.201903924.
- Kutin, Y., Cox, N., Lubitz, W., Schnegg, A., Rüdiger, O. (2019). In Situ EPR Characterization of a Cobalt Oxide Water Oxidation Catalyst at Neutral pH Catalysts 9(11), 926. https://doi.org/10.3390/catal9110926.
- Nehrkorn, J., Bonke, S.A., Aliabadi, A., Schwalbe, M., Schnegg, A. (2019). Examination of the Magneto-Structural Effects of Hangman Groups on Ferric Porphyrins by EPR Inorganic Chemistry 58(20), 14228-14237. https://doi.org/10.1021/acs.inorgchem.9b02348.
- Sidabras, J., Duan, J., Winkler, M., Happe, T., Hussein, R., Zouni, A., Suter, D., Schnegg, A., Lubitz, W., Reijerse, E.J. (2019) Extending electron paramagnetic resonance to nanoliter volume protein single crystals using a self-resonant microhelix Science Advances 5(10), eaay1394. https://doi.org/10.1126/sciadv.aay1394.
- Cheng, J., Liu, J., Leng, X., Lohmiller, T., Schnegg, A., Bill, E., Ye, S., Deng, L. (2019). A Two-Coordinate Iron(II) Imido Complex with NHC Ligation: Synthesis, Characterization, and Its Diversified Reactivity of Nitrene Transfer and C–H Bond Activation Inorganic Chemistry 58, 7634-6744. https://doi.org/10.1021/acs.inorgchem.9b01147.
- Zhao, G., Busser, G.W., Froese, C., Hu, B., Bohnke, S.A., Schnegg, A., Ai, Y., Wei, D., Wang, X., Peng, B., Muhler, M. (2019). Anaerobic Alcohol Conversion to Carbonyl Compounds Over Nanoscaled Rh-doped SrTiO3 under Visible Light The Journal of Physical Chemistry Letters 10, 2075–2080. https://doi.org/10.1021/acs.jpclett.9b00621.
- Nehrkorn, J., Veber, S.L., Zhukas, L.A., Novikov, V.N., Nelyubina, Y.V., Voloshin, Y.Z, Holldach, K., Stoll, S., Schnegg, A. (2018). Determination of Large Zero-Field Splitting in High-Spin Co(I) Clathrochelates Inorganic Chemistry 57(24), 15330-15340. https://doi.org/10.1021/acs.inorgchem.8b02670.
Group members
EPR Research Group @ MPI CEC
The EPR Research Group at MPI CEC employs electron paramagnetic resonance (EPR) spectroscopy to identify and characterise paramagnetic states relevant to energy conversion and storage processes. Special focus is devoted to catalytically active transition-metals and main group compounds as well as organic radicals. We develop and utilise state-of-the-art EPR spectrometers ranging from GHz to THz frequencies. Our spectrometers are capable of a wide repertoire of CW/pulsed multi-resonance and multi-frequency EPR methods for powder, crystal, solution and in-situ experiments.
Currently we are working in the following fields of research:
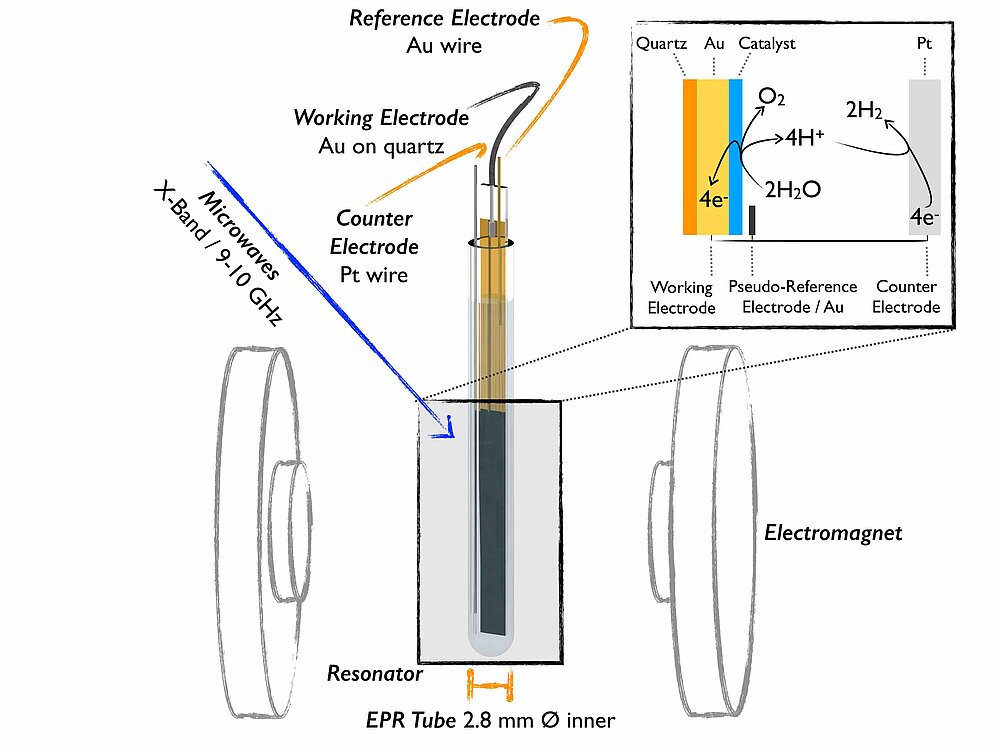
In-situ EPR
An understanding of catalyst function enables rational modification and improvement. Yet, the most reactive and catalytically active states are typically only formed under operational conditions. This motivates our group to develop methodology for in situ / operando studies that allow EPR characterisation of catalysts for energy conversion reactions. These studies require electrochemical measurements to be undertaken within the constraints of specific spectroscopic equipment, which we design and develop to ensure the conditions of analysis are truly relevant to the operational catalytic activity.
The applicability of EPR to any electrochemically derived/analysed species is that single electron redox steps frequently involve paramagnetic states. The exceptional sensitivity of spectro-electrochemical EPR (SEC EPR) to these states makes it powerful in examining the subtle, yet crucial, electronic effects that enable catalysis. EPR also allows selective analysis of paramagnetic oxidation states independent of diamagnetic species, thus providing selectivity that is unavailable with many other techniques - especially if the catalyst ground state is diamagnetic. In-situ EPR spectra are interpreted with extensive experiment-simulation comparisons and then correlated with catalytic measurements as well as with results from other techniques. SEC EPR is employed within DFG collaborative research centre (CRC) 247 for studies in paramagnetic states in Co oxide catalysts for alcohol oxidation reactions and within CRC 1487 for the characterization of high spin iron states in single atom catalysts for the oxygen reduction reactions.
Bonke, S. A.; Risse, T.; Schnegg, A.; Brückner, A. (2021) In situ electron paramagnetic resonance spectroscopy for catalysis. Nature Review Methods Primers, https://doi.org/10.1038/s43586-021-00031-4
Contact: Dr. Kaltum Abdiaziz
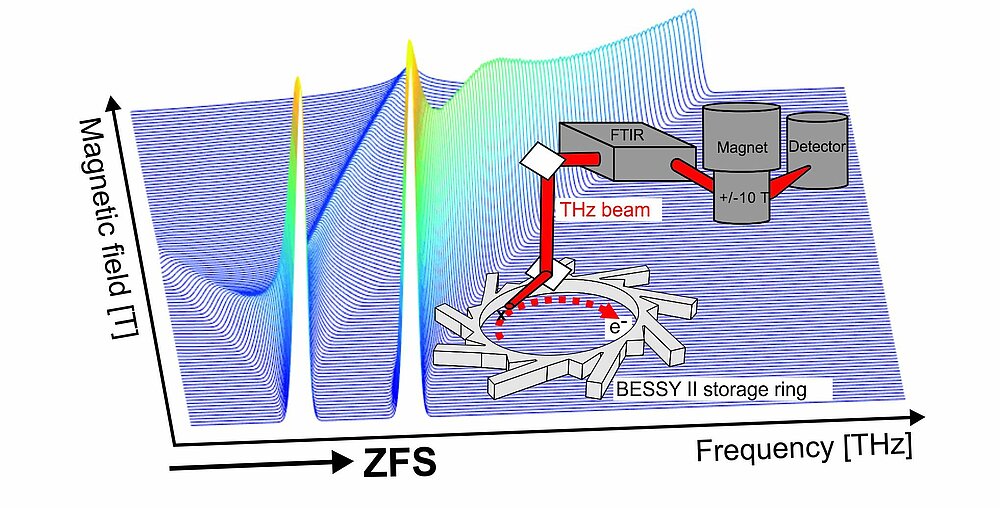
High-spin transition metal ion states
EPR characterisation of mono- and multi-nuclear high-spin states (electron spin, S > 1/2) in transition-metal ions targets the determination of their spin coupling parameters, in particular zero-field splittings and exchange couplings. The latter are sensitive probes of the metal ion’s coordination environment and electronic structure. In the case of a catalytically active ion, spin couplings provide unique information on its structure-function relationship. However, the highly desired spin couplings are oftentimes not accessible with standard 9.5 GHz EPR spectrometers. To bridge this gap we develop and apply advanced field and frequency domain EPR methods, covering the GHz to THz EPR-excitation energy range.
Lohmiller, T.; Spyra, C.-J.; Dechert, S.; Demeshko, S.; Bill, E.; Schnegg, A.; Meyer, F. (2022) Antisymmetric Spin Exchange in a μ-1,2-Peroxodicopper(II) Complex with an Orthogonal Cu–O–O–Cu Arrangement and S = 1 Spin Ground State Characterized by THz-EPR. JACS Au, https://doi.org/10.1021/jacsau.2c00139
Contact: Dr. Alexander Schnegg
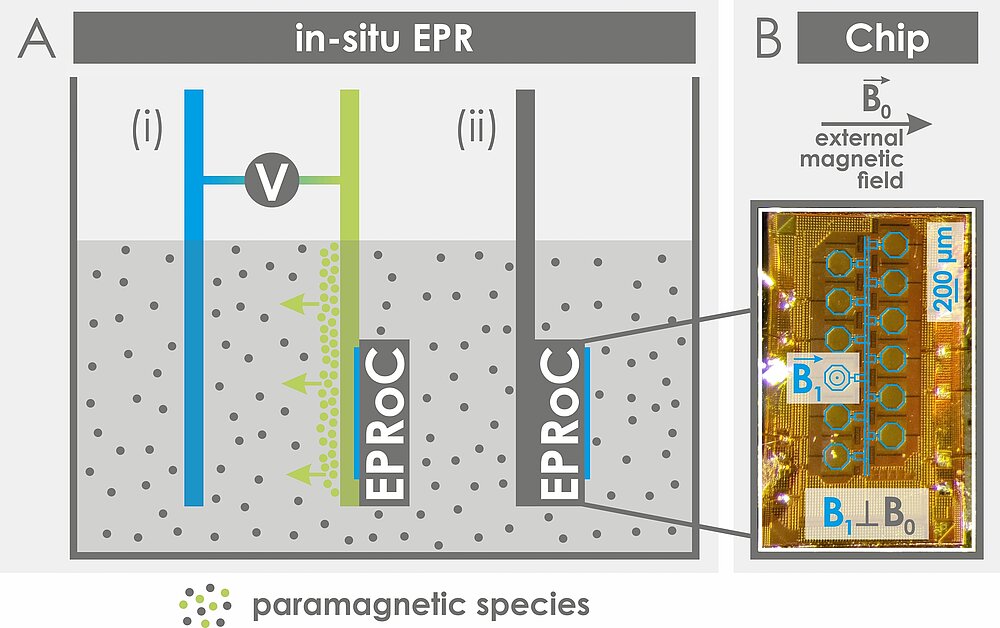
EPR-on-a-Chip (EPRoC)
EPRoC are mm-sized sensors that incorporate a microwave source and detector on a surface array allowing for a fundamental paradigm shift in EPR spectroscopy by facilitating in situ measurements of paramagnetic samples in miniaturized setups in a cost-efficient way. We are interested in integrating the EPRoC sensors, designed in the Anders Group (Universität Stuttgart), in an electrode for electrochemical experiments, alongside the characterization of paramagnetic states in liquid solutions, e.g. in electrochemical cells, batteries or reactors. Research with EPRoC sensors is funded by the Federal Ministry of Education and Research (Grant reference number: 03SF0565A).
Contact: Dr. Takuma Sato
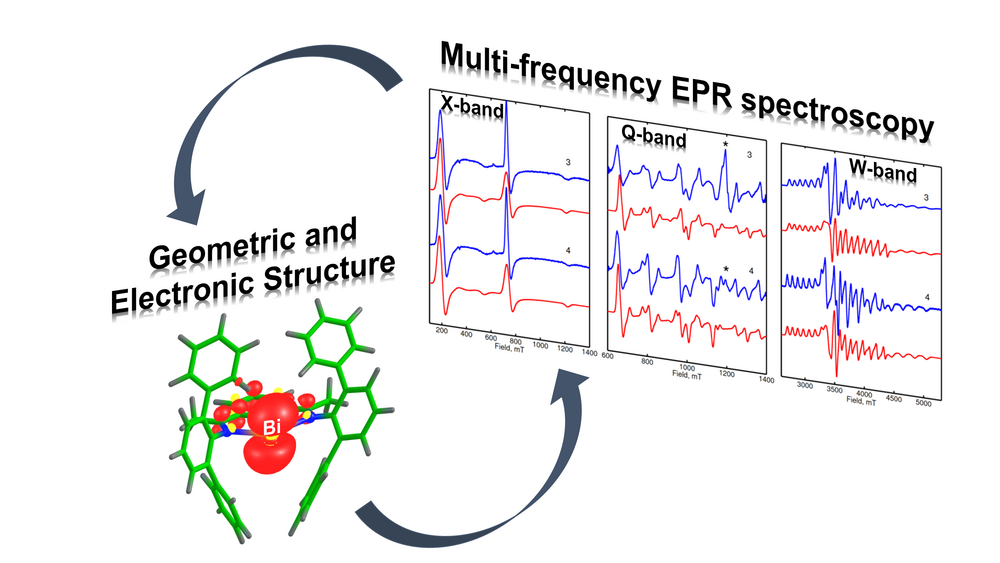
Active Site Characterization by Multi-frequency EPR and Hyperfine Spectroscopy
The identification and characterization of catalytically active sites occupy a central place in the study of catalysis. As the active sites often involve paramagnetic states, EPR spectroscopy is the method of choice to obtain exquisite details on their geometric and electronic structure. In our group, high-resolution multi-frequency (S-, X-, Q- and W-band) and multi-resonance EPR techniques are employed to provide such functional information in the different branches of catalysis ranging from homogenous, heterogeneous and single-site heterogeneous catalysis. By using hyperfine spectroscopies (HYSCORE, ENDOR and EDNMR) we monitor the nuclear spins from the first and second coordination sphere of the paramagnetic active sites. This allows us to shed light on the intimate features of the chemical bonding, which is crucial to understand the catalytic potential of active species. The calculation of the EPR parameters with state-of-the-art electronic structure quantum chemical methods translates the spectroscopic findings into microscopic structure of the catalyst's active site enabling structure–property correlations.
Yang, X.; Reijerse, E. J.; Bhattacharyya, K.; Leutzsch, M.; Kochius, M.; Nöthling, N.; Busch, J.; Schnegg, A.; Auer, A. A.; Cornella, J. (2022) Radical Activation of N–H and O–H Bonds at Bismuth(II). Journal of the American Chemical Society, https://doi.org/10.1021/jacs.2c05882
Lin, Y.-H.; Kutin, Y.; van Gastel, M.; Bill, E.; Schnegg, A.; Ye, S.; Lee, W.-Z. (2020) A Manganese(IV)-Hydroperoxo Intermediate Generated by Protonation of the Corresponding Manganese(III)-Superoxo Complex. Journal of the American Chemical Society, https://doi.org/10.1021/jacs.0c02756
Contact: Dr. Edward J. Reijerse and Dr. Paolo Cleto Bruzzese