Prof. Martin Muhler - Heterogeneous Redox Catalysis
- Prof. Dr. Martin Muhler
- Max Planck Fellow
- Heterogeneous Redox Catalysis
- +49 (0)234 32 287 54
- martin.muhler(at)cec.mpg.de
Short Bio
Martin Muhler studied chemistry at the Ludwig-Maximilians-Universität in Munich from 1980 to 1986. He moved to Berlin to the Fritz-Haber-Institute of the Max-Planck-Society (FHI) joining Prof. Dr. G. Ertl’s group as PhD student. He received his PhD in 1989 from the Freie Universität Berlin. He joined Haldor Topsoe A/S in Denmark as a postdoctoral fellow from 1989 to 1991. He returned to Prof. Ertl’s Department of Physical Chemistry as head of the group “Heterogeneous Catalysis” and finished his habilitation in Industrial Chemistry in 1996 (Technische Universität Berlin). In 1996 he was appointed full professor in Industrial Chemistry at the Ruhr-University Bochum, where he still is.
His research is focused on heterogeneous redox catalysis comprising selective reduction and oxidation, electrocatalysis and photocatalysis. Syngas catalysis is a major topic on which he has been working for more than 20 years. His number of publications is close to 400 (h-index of 51). He is a member of the International Advisory Boards of ChemCatChem, ChemSusChem, and the State Key Laboratory of Catalysis at the DICP in Dalian. He is the German representative in the European Federation of Catalysis Societies (EFCATS) and in the International Association of Catalysis Societies (IACS). Since 2014 he is the Chairman of the German Catalysis Society (GeCatS).
Publications
Publications
Selected Publications
- Büker, J., Muhler, M., Peng, B. (2023). Concepts of Heterogeneously Catalyzed Liquid-Phase Oxidation of Cyclohexene with <i>tert</i>-Butyl Hydroperoxide, Hydrogen Peroxide and Molecular Oxygen. CHEMCATCHEM, 15(1): e202201216. doi:10.1002/cctc.202201216.
- Deitermann, M., Haver, Y., Mei, B. T., Muhler, M. (2023). The Influence of the Reaction Conditions on the Photocatalytic Gas-Phase Conversion of Methanol with Water Vapor over Pt/SrTiO3 in a Continuously Operated Flow Reactor. Advanced Sustainable Systems, 2300329, pp. 1-10. doi:10.1002/adsu.202300329.
- Mockenhaupt, B., Schwiderowski, P., Jelic, J., Studt, F., Muhler, M., Behrens, M. (2023). High-Pressure Pulsing of Ammonia Results in Carbamate as Strongly Inhibiting Adsorbate of Methanol Synthesis over Cu/ZnO/Al2O3. Journal of Physical Chemistry C, 127, 3497-3505. doi:10.1021/acs.jpcc.2c08823.
- Geiss, J., Büker, J., Schulte, J., Peng, B., Muhler, M., Winterer (2023). LaCo1-XFeXO3 Nanoparticles in Cyclohexene Oxidation. The Journal of Physical Chemistry C, (127), 5029-5038.https://doi.org/10.1021/acs.jpcc.2c08644
- Tang, D., Shen, Z., Lechler, S., Lu, G., Yao, L., Hu, Y., Huang, X., Muhler, M., Zhao, G., Peng, B. (2023). Aerobic oxidative lactonization of diols at room temperature over defective titanium-based oxides in water. JOURNAL OF CATALYSIS, 418, 237-246. doi:10.1016/j.jcat.2023.01.025.
- Falk, T., Budiyanto, E., Dreyer, M., Büker, J., Weidenthaler, C., Behrens, M., Tüysüz, H., Muhler, M., Peng, B. (2022). Doping of Nanostructured Co3O4 with Cr, Mn, Fe, Ni, and Cu for the Selective Oxidation of 2-Propanol. ACS Applied Nano Materials, (5), 17783-17794. doi:10.1021/acsanm.2c03757.
- Schwiderowski, P., Ruland, H., Muhler, M. (2022). Current developments in CO(2 )hydrogenation towards methanol: A review related to industrial application. Current Opinion in Green and Sustainable Chemistry, 38(38): 100688, .doi:10.1016/j.cogsc.2022.100688.
- Schwiderowski, P., Stürmer, S., Muhler, M. (2022). Probing the methanol-assisted autocatalytic formation of methanol over Cu/ZnO/Al2O3 by high-pressure methanol and methyl formate pulses. REACTION CHEMISTRY & ENGINEERING, 7(10),2224-2230. doi:10.1039/d2re00185c.
- Geiss, J., Falk, T., Ognjanovic, S., Anke, S., Peng, B., Muhler, M., Winterer, M. (2022). Atom Pair Frequencies as a Quantitative Structure-Activity Relationship for Catalytic 2-Propanol Oxidation over Nanocrystalline Cobalt-Iron-Spinel. The Journal of Physical Chemistry C, 126(25), 10346-10358. doi:10.1021/acs.jpcc.2c00788.
- Xiang, W., Yang, N., Li, X., Linnemann, J., Hagemann, U., Rüdiger, O.,Heidelmann,M.; Falk, T., Aramani, M., DeBeer, S., Muhler, M.,Tschulik, K., Li, T.. (2022). 3D atomic-scale imaging of mixed Co-Fe spinel oxide nanoparticles during oxygen evolution reaction. Nature Communications, 13(179), 1-14. doi:10.1038/s41467-021-277.
- Büker, J., Angel, S., Salamon, S., Landers, J., Falk, T., Wende, H., Wiggers, H., Schulz, C., Muhler, M., Peng, B. (2022). Structure-activity correlation in aerobic cyclohexene oxidation and peroxide decomposition over CoxFe3-xO4 spinel oxides. Catalysis Science & Technology, (12), 3594-3605. doi:10.1039/d2cy00505k.
- Huang, X., Li, X., Xia, W., Hu, B., Muhler, M., Peng, B. (2022). Highly dispersed Pd clusters/nanoparticles encapsulated in MOFs via in situ auto-reduction method for aqueous phenol hydrogenation. Journal of Materials Science & Technology, (109), 167-175. doi:10.1016/j.jmst.2021.08.079.
- Hu, B., Warczinski, L., Li, X., Lu, M., Bitzer, J., Heidelmann, M., Eckhard, T., Fu,Q.,Schulwitz, J.,Mingshi M.M.L., Kleist, W., Hättig, C., Muhler, M., Peng ,P. (2021) Formic Acid-Assisted Selective Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran over Bifunctional Pd Nanoparticles Supported on N-Doped Mesoporous Carbon. Angewandte Chemie, International Edition in English, (60), 6807-6815. doi:10.1002/anie.202012816.
- Büker, J., Alkan, B., Chabbra, S., Kochetov, N., Falk, T., Schnegg, A., Schulz,C., Wiggers, H., Muhler, M., Peng, B. (2021) Liquid-Phase Cyclohexene Oxidation with O-2 over Spray-Flame-Synthesized La1-xSrxCoO3 Perovskite Nanoparticles. SI, 27(68), 16912-16923. doi:10.1002/chem.202103381.
- Brix, A. C., Morales, D. M., Braun, M., Jambrec, D., Junqueira, J. R. C., Cychy, S., Seisel, S.,Masa, J., Muhler, M., Andronescu, C., Schuhmann, W., (2021). Electrocatalytic Oxidation of Glycerol Using Solid-State Synthesised Nickel Boride: Impact of Key Electrolysis Parameters on Product Selectivity. ChemElectroChem, (8), 2336-2342. doi:10.1002/celc.202100739.
- Hu, Y., Shen, Z., Li, B., Li, S., Yue, J., Zhao, G., Muhler, M., Wang, X. (2021) Solvent Effects on Photocatalytic Anaerobic Oxidation of Benzyl Alcohol over Pt-Loaded Defective SrTiO3 Nanoparticles. ACS Applied Nano Materials, 4(9), 9254-9264. doi:10.1021/acsanm.1c01750.
- Falk, T., Anke, S., Hajiyani, H., Saddeler, S., Schulz, S., Pentcheva, R., Peng, B., Muhler, M. (2021) Influence of the particle size on selective 2-propanol gas-phase oxidation over Co3O4 nanospheres. Catalysis Science & Technology, (11) 7552-7562. doi:10.1039/d1cy00944c.
- Sikdar, N., Schwiderowski, P., Medina, D., Dieckhöfer, S., Quast, T., Brix, A. C., Cychy, S., Muhler, M., Masa, J., Schuhmann, W. (2021) Construction of a Poly(anthraquinone Sulfide)/Carbon Nanotube Composite with Enhanced Li-ion Storage Capacity. CHEMELECTROCHEM, 8(9), 1685-1693. doi:10.1002/celc.202100374.
- Falk, T., Budiyanto, E., Dreyer, M., Pflieger, C., Waffel, D., Büker, J., Weidenthaler, C., Ortega, K. F., Behrens, M., Tueysuez, H., Muhler, M., Peng, B. (2021) Identification of Active Sites in the Catalytic Oxidation of 2-Propanol over Co1+xFe2-xO4 Spinel Oxides at Solid/Liquid and Solid/Gas Interfaces. ChemCatChem, (13), 2942-2951. doi:10.1002/cctc.202100352.
- Zhao, G., Bonke, S. A., Schmidt, S., Wang, Z., Hu, B., Falk, T., Hu, Y., Rath, T ., Xia, W ., Peng, B., Schnegg, A., Weng, Y., Muhler, M. (2021) Highly Efficient and Selective Aerobic Oxidation of Cinnamyl Alcohol under Visible Light over Pt-Loaded NaNbO3 Enriched with Oxygen Vacancies by Ni Doping. ACS Sustainable Chemistry & Engineering, 9(15), 5422-5429. doi:10.1021/acssuschemeng.1c00460.
- Büker, J., Huang, X., Bitzer, J., Kleist, W., Muhler, M., Peng, B. (2021) Synthesis of Cu Single Atoms Supported on Mesoporous Graphitic Carbon Nitride and Their Application in Liquid-Phase Aerobic Oxidation of Cyclohexene. ACS CATALYSIS, (11), 7863-7875. doi:10.1021/acscatal.1c01468.
- R. Naumann d’Alnoncourt, X. Xia, J. Strunk, E. Löffler, O. Hinrichsen, M. Muhler, “The Influence of Strongly Reducing Conditions on Strong Metal-Support Interactions in Cu/ZnO Catalysts Used for Methanol Synthesis”, Phys. Chem. Chem. Phys. 8 (2006) 1525-1538.
- S. Kaluza, M.K. Schröter, R. Naumann d’Alnoncourt, T. Reinecke, M. Muhler, “High Surface Area ZnO Nanoparticles via a Novel Continuous Precipitation Route”, Adv. Funct. Mater. 18 (2008) 3670-3677
- S. Kundu, T. C. Nagaiah, W. Xia, Y. Wang, S. van Dommele, J. H. Bitter, M. Santa, G. Grundmeier, M. Bron, W. Schuhmann, M. Muhler, “Electrocatalytic activity and stability of nitrogen-containing carbon nanotubes in the oxygen reduction reaction”, J. Phys. Chem. C 113 (2009) 14302-14310.
- B. Graf, H. Schulte, M. Muhler, “The Formation of Methane over Iron Catalysts Applied in Fischer-Tropsch Synthesis: A Transient and Steady State Kinetic Study”, J. Catal. 276 (2010) 66-75.
- C. Li, A. Zhao, W. Xia, C. Liang, M. Muhler, „Quantitative studies on the oxygen and nitrogen functionalization of carbon nanotubes in the gas phase”, J. Phys. Chem. C 116 (2012) 20930−20936.
- S. Kundu, T. Chikka Nagaiah, X. Chen, W. Xia, M. Bron, W. Schuhmann, M. Muhler, „Synthesis of an improved hierarchical carbon-fiber composite as a catalyst support for platinum and its application in electrocatalysis”, Carbon 50 (2012) 4534-4542.
- K. Kähler, M.C. Holz, M. Rohe, A.C. van Veen, M. Muhler, “Methanol oxidation as probe reaction for active sites in Au/ZnO and Au/TiO2 catalysts”, J. Catal. 299 (2013) 162-170.
- H. Düdder, K. Kähler, B. Krause, K. Mette, S. Kuhl, M. Behrens, V. Scherer, M. Muhler, „The role of carbonaceous deposits in the activity and stability of Ni-based catalysts applied in the dry reforming of methane”, Catal. Sci. Technol. 4 (2014) 3317-3328.
- Zhao, J. Masa, W. Xia, A. Maljusch, M.-G. Willinger, G. Clavel, K. Xie, R. Schlögl, W. Schuhmann, M. Muhler, “Spinel Mn-Co oxide in N-doped Carbon Nanotubes as Bifunctional Electrocatalyst Synthesized by Oxidative Cutting”, J. Am. Chem. Soc. 136 (2014) 7551–7554.
- J. Anton, J. Nebel, H. Song, C. Froese, P. Weide, H. Ruland, M. Muhler, S. Kaluza, “Structure–activity relationships of Co-modified Cu/ZnO/Al2O3 catalysts applied in the synthesis of higher alcohols from synthesis gas”, Appl. Catal. A: General 505 (2015) 326-333.
- R. Chetty, K. K. Maniam, W. Schuhmann, M. Muhler, “Oxygen-Plasma-Functionalized Carbon Nanotubes as Supports for Platinum-Ruthenium Catalysts Applied in Electrochemical Methanol Oxidation”, ChemPlusChem 80 (2015) 130-135.
- W. Dong, S. Reichenberger, S. Chu, P. Weide, H. Ruland, S. Barcikowski, P. Wagener, M. Muhler, (2015)The effect of the Au loading on the liquid-phase aerobic oxidation of ethanol over Au/TiO2 catalysts prepared by pulsed laser ablation”, J. Catal. 330 (2015) 497-506.
- J. Anton, J. Nebel, H. Song, C. Froese, P. Weide, H. Ruland, M. Muhler, S. Kaluza, “The effect of sodium on the structure–activity relationships of cobalt-modified Cu/ZnO/Al2O3 catalysts applied in the hydrogenation of carbon monoxide to higher alcohols”, J. Catal. 335 (2016) 175–186.
Gruppenmitglieder
Heterogeneous Redox Catalysis
Three-Phase Catalysis under Mild Conditions
The Max Planck Fellow group “Heterogeneous Redox Catalysis” (<link http: www.techem.rub.de>
) performs fundamental research in the area of heterogeneous redox catalysis in the liquid phase under mild conditions and aims at developing catalysts based on mechanistic insight. The scientific challenge is the elucidation of the reaction mechanisms and the interplay of the reactants with the complex surface chemistry of heterogeneous catalysts on the atomic level. Heterogeneous catalysts usually consist of many phases and components, often present as nanoparticles or as X-ray amorphous layers on bulk supports. For many catalysts the chemical reactivity of supported metallic or oxidic nanoparticles is influenced by strong interactions with the support.The reaction conditions of thermal redox catalysis experiments are chosen at temperatures below 200 °C in the liquid phase to guarantee conservation of the kinetic control of specific materials properties such as defects that have been deliberately adjusted by targeted catalyst synthesis. Reduction catalysis focuses on the hydrodeoxygenation of polyols such as glycerol, whereas oxidation catalysis comprises the selective oxidation of short chain alcohol and the challenging C-H bond activation of hydrocarbons. Low temperatures also exclude the Mars – van Krevelen mechanism by avoiding bulk diffusion of oxygen anions in oxides. In addition to thermal catalysis, electrocatalytic reactions such as the oxygen evolution reaction (OER) and electrochemical alcohol oxidation are also investigated. Liquid phase oxidation and electrocatalysis require a deeper understanding of solvation-related phenomena. Corrosion is a common phenomenon leading to catalyst deactivation or dissolution and re-deposition equilibria.
Catalytic Reduction
Glycerol is an attractive biomass feedstock due to its relatively low cost and ample availability as a by-product of biodiesel production. Glycerol can be converted to high value-added oxygenated chemicals such as propanediols via hydrodeoxygenation. The aqueous phase deoxygenation of glycerol to propanediols requires selectively cleaving just one of the C–O bonds, but preserving the C–C bonds. It occurs catalytically on bifunctional catalysts via consecutive dehydration and hydrogenation in the presence of H2 at moderate temperatures and relatively high pressures. By far, catalyst exploration has been mainly focused on noble metal-based catalysts such as Pt/Al2O3,[1,2] and therefore the utilization of low-cost Cu-based catalyst is of high interest.
Catalytic Oxidation
Oxidation reactions are investigated with molecular oxygen as the preferred oxidant as well as with alternative oxidants such as hydrogen peroxide and tert-butyl hydroperoxide (TBHP) as oxygen donor. Employed catalysts include both precious metal-based catalysts such as Au/TiO2 and Pd/CNT, or base metal oxides such as Co-containing spinels and perovskites. The reactions comprise liquid-phase oxidation of short-chain alcohols,[3,4] the oxidation of biomass-related 5-hydroxymethylfurfural (5-HMF) to 2,5-furan dicarboxylic acid (2,5-FDCA), as well as on the challenging C-H bond activation of aliphatic or aromatic hydrocarbons.
For example, we have recently studied the selective oxidation of ethanol in the liquid phase with Pd nanoparticles supported on carbon nanotubes (CNTs).[4] The characterization of the surface and bulk properties combined with the catalytic tests indicate the dissolution and re-deposition of Pd species under reaction conditions. Nitrogen-doped carbon nanotubes (NCNTs) act as an excellent support for the Pd catalyst system by efficiently stabilizing and recapturing the Pd species resulting in high activity and selectivity to acetic acid.
The abovementioned reactions are primarily performed in the batch mode, that is, reactions in autoclaves (see Figure 1 and 2) to screen the catalysts and to optimize reaction conditions. In addition, continuously operated reactors such as the H-Cube Pro TM system (see Figure 3), having many advantages like higher interfacial area, better mass / heat transfer, safe and easy operation, will be utilized for systematic kinetic studies. In close collaboration with Prof. Dr.-Ing. Marcus Gruenewald in Chemical Engineering at the Ruhr-University Bochum (http://www.fluidvt.rub.de), micro-structured reactors will be developed and operated continuously.
Electrocatalysis
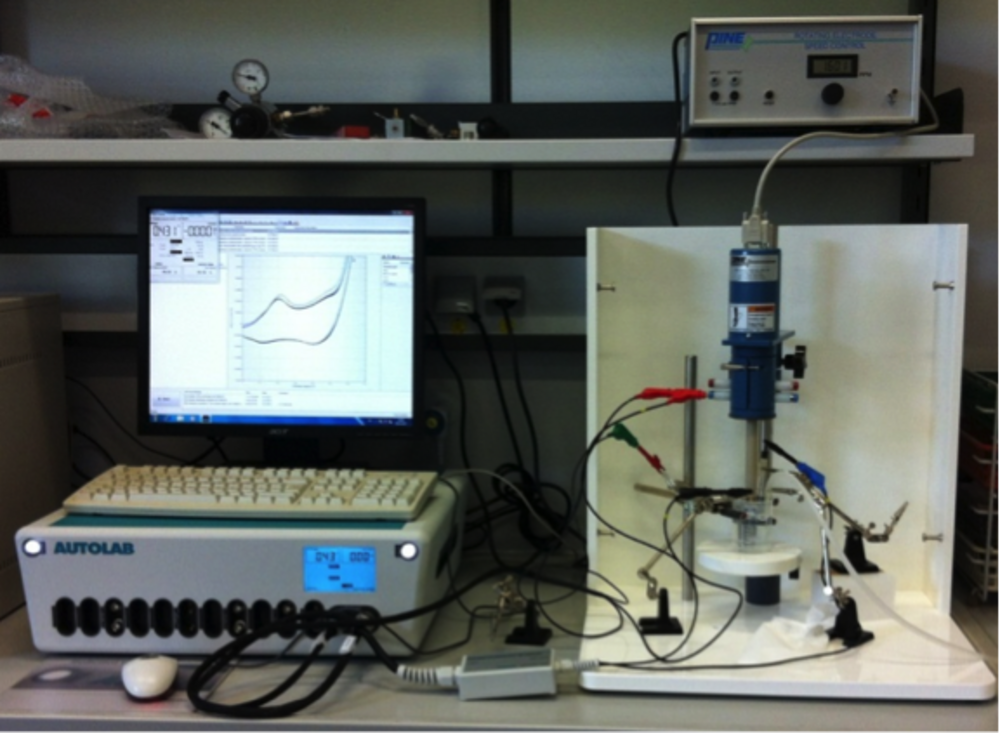
Electrocatalysis combines heterogeneous catalysis and electrochemistry. Thermal and electrochemical processes involving coupled electron and proton transfer and radicals as intermediates can be studied using the same heterogeneous catalyst provided that the support has sufficient electrical conductivity. Electrochemical alcohol oxidation reactions as well as the attractive OER are our main interest in this field, and most measurements are carried out using an electrochemical workstation (see Figure 4). In-depth electrocatalytic studies are performed in close collaboration with Prof. Dr. Wolfgang Schuhmann (http://www.rub.de/elan) at the Ruhr-University Bochum using all state-of-the-art electrochemical methods including nanoelectrodes (scanning electrochemical microscope, SECM).
For example, we have recently demonstrated the improved utilization of palladium as anode catalyst for ethanol oxidation by exploiting the strong interaction between Pd nanoparticles and NCNTs as support [5]. 0.85 wt% Pd/NCNTs achieved a specific current density of 517 A/gPd. The electrocatalytic performance deteriorated only gradually and catalysis was sustained for at least 80 h.
ATR-IR Spectroscopy
In situ attenuated total reflection infrared spectroscopy (ATR-IR, see Figure 5) is used to investigate reaction mechanisms of liquid-phase redox reactions. ATR-IR is ideally suited for studying molecular vibrations at the solid-liquid interface, because the evanescent wave is restricted to the region near the interface of the internal reflection element, thereby minimizing the contribution from the solvent. The in situ ATR-IR investigation of a heterogeneous solid-liquid catalytic reaction provides information about the catalytic surface and the absorbed species on the catalyst surfaces such as reactants, products, and long-lived intermediates. These investigations are supported by in situ Raman spectroscopy studies in Prof. Schuhmann’s group.
Our research also contributes to the Cluster of Excellence RESOLV (http://www.ruhr-uni-bochum.de/solvation) aiming at a deeper understanding of solvation-related phenomena.
References
[1] A. Wawrzetz, B. Peng, A. Hrabar, A. Jentys, A. A. Lemonidou, J. A. Lercher, J. Catal. 269 (2010) 411.
[2] B. Peng, C. Zhao, I. Mejía-Centeno, G. A. Fuentes, A. Jentys, J. A. Lercher, Catal. Today 183 (2012) 3.
[3] W. Dong, S. Reichenberger, S. Chu, P. Weide, H. Ruland, S. Barcikowski, P. Wagener, M. Muhler, J. Catal. 330 (2015) 497.
[4] W. Dong, P. Chen, W. Xia, P. Weide, H. Ruland, A. Kostka, K. Köhler, M. Muhler, ChemCatChem (2016) 8, 1269 – 1273.
[5] D. Hiltrop, J. Masa, A. Maljusch, W. Xia, W. Schuhmann, M. Muhler, Electrochemistry Communications 63 (2016) 30.